All published articles of this journal are available on ScienceDirect.
Meniscal Scaffolds - Preclinical Evidence to Support their Use: A Systematic Review
Abstract
Arthroscopic meniscal treatment is the most common procedure performed in the orthopedic practice. Current management of meniscal pathology relies on different therapeutic options, ranging from selective meniscectomy, suturing, and to meniscal replacement by using either allografts or scaffolds. The progresses made in the field of regenerative medicine and biomaterials allowed to develop several meniscal substitutes, some of those currently used in the clinical practice. Before reaching the clinical application, these devices necessarily undergo accurate testing in the animal model: the aim of the present manuscript is to systematically review the scientific evidence derived by animal model results for the use of meniscal scaffolds, in order to understand the current state of research in this particular field and to identify the trends at preclinical level that may influence in the near future the clinical practice.
Thirty-four papers were included in the present analysis. In 12 cases the meniscal scaffolds were used with cells to further stimulate tissue regeneration. With the exception of some negative reports regarding dacron-based scaffolds, the majority of the trials highlighted that biomaterials and bio-engineered scaffolds are safe and could play a beneficial role in stimulating meniscal healing and in chondral protection. With regard to the benefits of cell augmentation, the evidence is limited to a small number of studies and no conclusive evidence is available. However, preclinical evidence seems to suggest that cells could enhance tissue regeneration with respect to the use of biomaterials alone, and further research should confirm the translational potential of cell-based approach.
INTRODUCTION
The first arthrotomic procedure on meniscus was performed by Robert Annandale at the end of the 19th century [1], and from that moment onwards meniscal surgery has become common in the everyday orthopaedic practice. For the most part of the 20th century the main procedure on meniscus was removal of the damaged tissue, which was further diffused by the advent of arthroscopy that contributed to minimize surgical trauma and accelerate knee function recovery. It took almost one hundred years to achieve, in 1984, the first meniscal allograft transplantation, which opened the era of biological replacement [2]. Nowadays, the current management of meniscal pathology is focused on preserving the meniscal tissue as much as possible, in order to prevent the early onset of osteoarthritis (OA) in the affected compartment [3, 4]. To this purpose, beyond meniscal allografts, which are indicated in case of previous total or sub-total meniscectomy, new biomaterials have been brought into clinical practice: these scaffolds are used to replace partial meniscal defects [5], since it has been demonstrated that even a partial meniscectomy is able to alter joint biomechanics, determining an over-load in the affected compartment [3]. In this perspective, the possibility of using biomaterials able to stimulate meniscal tissue regeneration represents an innovative approach that could prevent patients from receiving more invasive surgery at young age. In fact, despite the costs linked to the use of such biomaterials, the possibility of preventing the onset of an early degenerative articular pathology is surely attractive and not only from the patient perspective, since it could also determine, on a large scale, a reduction in the expenses afforded by Health Systems to manage a chronic and invalidating condition such OA. To this purpose, it is useful to underline that more than 500,000 arthroscopies are performed every year to address meniscal pathology [5] and, therefore, it is easy to understand that the clinical implication and societal impact of novel regenerative procedures could be huge.
At present times there are two meniscal substitutes used in clinical practice: the first one is a collagen based meniscal implant, whereas the second and more recent one is made of polyurethane and polycaprolactone. Both of them have been investigated in a number of clinical trials, that revealed the safety of these implants and also their capability of improving knee functional status up to the possibility of resuming sport practice [6-8]. Imaging and, in some cases, arthroscopic evaluation confirmed the potential of these bio-engineered devices to stimulate meniscal tissue regeneration, although not in a complete manner. In some trials, these meniscal scaffolds have also been applied, together with cartilage treatments and osteotomies, to perform a biological knee reconstruction as salvage procedure in patients affected by unicompartmental OA and otherwise doomed to metal resurfacing [9]. Currently, this particular area of research, which is particularly based on biomaterials and bio-engineering, is moving forward to develop new products that could promote a better tissue regeneration and determine superior and longer-lasting clinical outcome.
The aim of the present manuscript is to systematically review the scientific evidence for the use of meniscal scaffolds derived by animal model results, in order to understand the current state of research in this particular field, underlining in particular the materials that have been tested, the outcomes in terms of meniscal regeneration, and the role played by cellular augmentation. Identifying the trends emerged at preclinical level that may lead to better understand the evolution in this particular area of research and shed light on the solutions that might be introduced in clinical practice in the near future.
MATERIALS AND METHODS
A systematic review of the literature was performed on the application of meniscal scaffolds / meniscal bio-engineered substitutes in the animal model. The search was made on the PubMed database on March 1st, 2015 using the following formula: (meniscal scaffold or meniscal substitute or meniscal replacement or meniscal biomaterial) AND (animal or horse or pig or goat or sheep or bovine or ovine or rat or rabbit or monkey or dog or primate). The screening process and analysis were performed separately by 2 independent researchers.
First, the articles were screened by title and abstract. The following inclusion criteria for relevant articles were used during the initial screening of titles and abstracts: reports of any level of evidence, written in the English language, with no time limitation, on the application of meniscal scaffold/meniscal bio-engineered substitutes in the animal model. Exclusion criteria were: articles written in other languages, human trials, and reviews. In the second step, the full texts of the selected articles were screened, with further exclusions according to the previously described criteria. Reference lists from the selected papers were also screened. Relevant data were then extracted and collected in a single database with consensus of the two observers to be analyzed for the purposes of the present manuscript. At the end of the process, 34 papers fulfilled the selection criteria and have been described in the following paragraphs (Fig. 1).
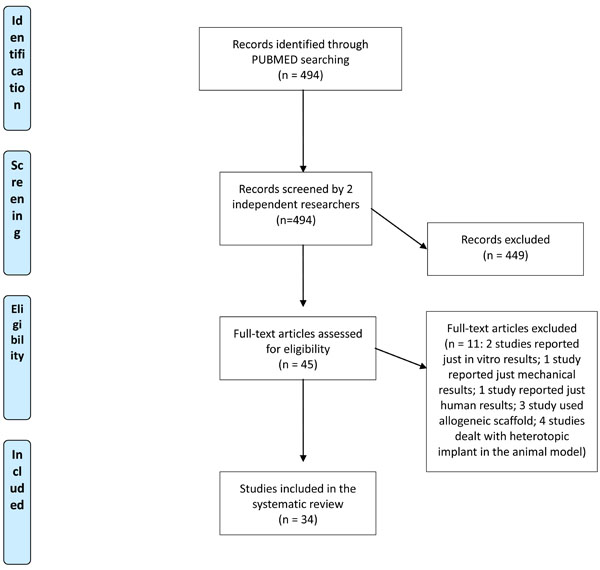
Flow Diagram describing the papers selection process.
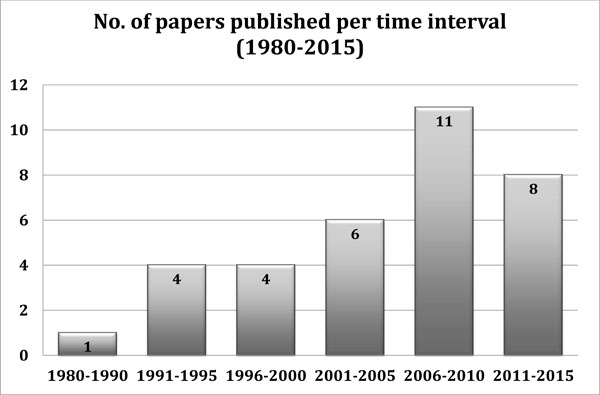
Numbers of papers published per time interval from 1980 up to today. (Note that the data for 2011-2015 period are still provisional).
RESULTS
A total of 34 papers fulfilled the inclusion criteria and were analyzed. The first paper was published in 1983 and, over the years, it was possible to observe an increasing interest by scientists in this particular area of musculoskeletal regenerative medicine (Fig. 2).
With regard to the animal models used, 1 paper dealt with the goat model, 1 paper with pig, whereas 8 papers with sheep, 13 with the rabbit, 10 with the dog, and 1 with both dog and pig model respectively. Furthermore, in 12 papers, cells (from different sources) were tested together with the meniscal scaffolds. Results will be described according to the animal model used and, when applicable, the particular cellular augmentation used will be also discussed.
A summary of all the studies included in the present review has been reported in Table 1.
Synopsis of animal trials dealing with the application of meniscal scaffolds.
| Author, Journal and Year | Animal Model |
Experiment Condition |
Biomaterial | Cells (If Added) | Additional Factors | Study Design | Follow-Up | Results |
|---|---|---|---|---|---|---|---|---|
| Zhang H, Clin Orthop Relat Res. 2009 [10] |
Goat | Full-thickness meniscal defect in avascular area | Calcium alginate gel | Bone marrow stromal cells | Insulin-like growth factor 1 (hIGF-1) introduced into the cells by gene transfection process | Transfected cells + scaffold Non-transfected cells + scaffold Scaffold alone Empty defect |
4, 8 and 16 weeks | Defects filled completely with the new meniscal tissue only for the cells transfected with hIGF-1 + scaffold group. Larger fibro-chondrocytes number and higher percentage of cartilage-like tissue for transfected cells group. |
| Weinand C, Am J Sports Med. 2006 [11] |
Pig | Bucket-handle lesion in the medial meniscus | Vicryl mesh scaffold | Autologous and allogeneic chondrocytes | - | Scaffold + autologous chondrocytes Scaffold + allogenic chondrocytes Scaffold alone Empty defect sutured Empty defect no treatment |
12 weeks | In allogeneic group 1 of 8 meniscus completely healed. In autologous group 2 of 9 meniscus completely healed. Other meniscus partially healed in both cell-seeded groups. |
| Martinek V, Arch Orthop Trauma Surg 2006 [13] |
Sheep | Total medial meniscectomy | Collagen scaffold | Pre-cultured Autologous chondrocytes | - | Scaffold+ autologous chondrocyte Scaffold alone Empty defect |
3 weeks and 3 months |
Enhanced vascularization, accelerated scaffold re-modelling, higher content of extra-cellular matrix and lower cell number were noted in the pre-seeded meniscus in comparison with non-seeded controls. |
| Chiari C, Osteoarthritis Cartilage 2006 [14] |
Sheep | Total and partial meniscus replacement | Hyaluronic acid and polycaprolactone composite | - | - | Meniscus substitute in partial meniscus replacement Meniscus substitute in total meniscus replacement Empty control |
6 weeks | Neo-meniscus tissue ingrowth Tissue integration between the original meniscus and the implant Cartilage degeneration occurred in the joints. |
| Kon E, Tissue Eng Part A 2008 [15] |
Sheep | Total medial meniscectomy | Hyaluronic acid and polycaprolactone composite | Autologous chondrocytes | Two fixation techniques: with or without trans-tibial horn fixation | Cell-seeded scaffolds Cell-free scaffolds Empty controls |
6 months | Superior fibrocartilage formation in the cell-seeded group. Better implant macroscopic appearance and integrity when the scaffold was fixed without trans-tibial horn suture. |
| Kon E, Tissue Eng Part A 2012 [16] |
Sheep | Total medial meniscectomy | Hyaluronic acid and polycaprolactone composite | Autologous chondrocytes | - | Cell-seeded scaffolds Cell-free scaffolds Empty controls |
12 months | Cell-seeded scaffold had better tissue regeneration capacity with more fibrocartilaginous tissue. Chondroprotective effect of the scaffold, although no difference was documented between cell-seeded and cell-free groups. |
| Kelly BT, Am J Sports Med. 2007 [17] |
Sheep | Total lateral Meniscectomy | Hydrogel meniscal implant | - | - | Hydrogel meniscal group Lateral meniscectomy group Meniscal allograft transplant |
2, 4 and 12 months | Promising results for hydrogel meniscal implants seen at early time points follow-up. However, at 12 months follow-up significant cartilage degeneration and implant failure for hydrogel meniscal group compared to allograft transplantation. |
| Maher SA, Arthroscopy 2010 [18] | Sheep | Partial lateral meniscectomy | Porous polyurethane scaffold | - | - | Scaffold group Defect untreated |
3, 6 and 12 months | Cartilage preserved under the implanted scaffold, with comparable defect filling between treatment groups (significant self-healing in partial meniscectomy model). |
| Zur G, Knee Surg Sports Traumatol Arthrosc. 2011 [19] |
Sheep | Total medial meniscectomy | Kevlar-reinforced polycarbonate-urethane (PCU) | - | - | Scaffold group Un-operated knee control |
3 and 6 months | No significant differences in terms of cartilage degeneration between scaffold and control group. |
| Gruchenberg K, Knee Surg Sports Traumatol Arthrosc 2014 [20] |
Sheep | Partial medial meniscectomy | Silk fibroin scaffold | - | - | Scaffold group Empty defect |
6 months | No inflammation occurred in scaffold implanted group. No significant differences in cartilage degeneration between the scaffold and sham group. Loss of the implant in 3 of 9 cases in scaffold group Mechanical properties of the scaffold similar to the native meniscal tissue. |
| Sommerlath K, Am J Sports Med. 1992 [21] |
Rabbit | Total meniscectomy and Incised meniscus | Dacron prosthesis with polyurethane coating | - | - | Prosthesis group Incised meniscus group Resected meniscus group |
3 months | Cartilage degeneration presented in 70% of the prosthesis implanted group, compared to only 25% in the group with meniscal incision No positive results regarding healing tissue for prosthesis group compared to incised meniscus or sham operated group |
| Sommerlath K, Clin Orthop Relat Res. 1993 [22] |
Rabbit | Incised meniscus and total meniscectomy | Dacron meniscus prosthesis | - | - | Prosthesis group Incised meniscus Resected meniscus |
3 months | Rare ingrowth but stable fixation of the prosthesis Prostheses implanted group showed the same incidence and severity of OA as the resected meniscus group |
| Messner K Biomaterials. 1993 [23] |
Rabbit | Total meniscectomy | Dacron and Teflon prosthesis | - | - | Dacron implant group Teflon implant group Resected meniscus group Non operated group |
3 months | Cartilage degeneration observed for both implants No positive results for implants (same results as the resected meniscus group) |
| Testa Pezzin AP, Artif Organs. 2003 [24] |
Rabbit | Total medial meniscectomy | Poly(p-dioxanone)/poly(L-lactic acid), PPD/PLLA | - | - | Scaffold group Empty defect |
3, 6, 12, and 14 weeks |
Better tissue regeneration in scaffold implanted group compared to control group (empty defect) |
| Kobayashi M, Biomaterials 2003 [25]; Biomed Mater Eng. 2004 [26]; Biomaterial 2005 [27] | Rabbit | Total lateral meniscectomy | Polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus | - | - | Scaffold group Lateral meniscectomy group |
6 months, 1, 1.5 and 2 years | Good results for scaffold implanted group in terms of cartilage degeneration rate OA changes occurred in lateral meniscectomy group Neither dislocation nor breakage of scaffold observed |
| Angele P, J Biomed Mater Res 2008 [28] | Rabbit | Resection of the pars intermedia medial meniscus | Hyaluronian/Gelatin composite scaffold | Autologous BMSCs | Preculture of scaffolds in chondrogenic medium for 14 days | Scaffold alone Scaffold + BMSCs Defect untreated |
12 weeks | Predominant fibrous tissue in empty and scaffold alone groups Meniscus-like fibrocartilage and greater cross-sectional width in pre-cultured implants |
|
Zellner J, J Biomed Mater Res A. 2010 [29] |
Rabbit | 2 mm defects in the avascular zone of the meniscus | Hyaluronan-collagen composite | Autologous MSCs Autologous bone marrow PRP |
Preculture of scaffolds in chondrogenic medium for 14 days | Scaffold+ MSCs (pre-cultured in chondrogenic medium or not) Scaffold + bone marrow Scaffold +PRP Scaffold alone Empty defect |
12 weeks | In all groups except cell-free implants, improvement in healing of the defect. Non pre-cultured MSCs in the scaffold stimulated the regeneration of meniscus-like tissue. Pre-cultured MCSs showed only partially integration with the native meniscus. |
| Zellner J, J Biomed Mater Res B Appl Biomater. 2013 [30] |
Rabbit | Longitudinal meniscal tear in the avascular zone | Hyaluronan-collagen composite matrix | Autologous MSCs PRP |
Scaffold with MSCs preculture in chondrogenic medium for 14 days | Scaffold + MSCs (pre-cultured in chondrogenic medium or not) Scaffold + PRP Scaffold alone Empty defect Sutured defect |
6 and 12 weeks | Implantation with scaffold + MSCs initiated. Fibrocartilage-like repair tissue with better integration and biomechanical properties when pre-cultured MSCs were used. |
|
Kang SW Biomed Mater Res A. 2010 [31] |
Rabbit | Total meniscectomy model | Polyglycolic acid (PGA) fiber meshes | Allogeneic meniscal cells expanded in vitro | - | scaffold + allogeneic meniscal cells | 6, 10 and 36 weeks | Regeneration of fibrocartilage with maintained original shape and structure of neomeniscus. Biochemical and mechanical analysis differed from native meniscus. |
| Esposito AR, Biores Open Access. 2013 [32] |
Rabbit | Total medial meniscectomy | poly(L-co-D,L-lactic acid)/poly(capro-lactone-triol) scaffold | Rabbits, fibro-chondrocytes | Fibrochondrocytes preseeded on the scaffold expanded in vitro for 21 days | Scaffold + cells Scaffold without cells Empty defect |
12 and 24 weeks | Good integration of the implanted scaffold into native tissue. No rejection, infection and inflammation observed. Better results in generating of fibro-cartilaginous tissue towards cell-seeded scaffold compare to scaffolds without cells. |
| Oda S, J Biomaterial Appl. 2015 [33] | Rabbit | Partial meniscectomy (2 mm defect anterior part of medial meniscus) |
Type I Collagen scaffold | Infrapatellar fat pad | - | Scaffold alone Scaffold + fat pad Empty defect |
8 weeks | Meniscal damage in untreated defects. Maintained surface area, higher scores in scaffold + fat pad group. Collagen staining similar to healthy meniscus in scaffold + fat pad group. |
| Toyonaga T, Clin Orthop Relat Res. 1983 [34] |
Dog | Medial meniscectomy | Teflon-net | - | - | Scaffold Empty defect |
12 weeks | Gross appearance similar to native meniscus; cell ingrowth into the scaffold. |
| Stone KR, Am J Sports Med. 1992 [12] |
Dog and pig | 80% subtotal resection of the medial meniscus | Collagen-based scaffold | - | - | Scaffold group Resected meniscus group Re-implantation of autologous meniscus group |
3, 6, 9 and 12 months | Scaffold compatible with meniscal fibrochondrocyte growth in dogs. No meniscal regeneration in pigs. |
| Klompmaker J, Biomaterials. 1996 [36] |
Dog | Longitudinal lesion | Porous polyurethane | - | - | Scaffold group Empty defect Non operated group |
From 2 to 52 weeks | 2/3 of defects healed Newly formed fibrocartilage observed for scaffold implanted group. Controls showed repair only with fibrous tissue. |
| Klompmaker J, Biomaterials. 1996 [37] |
Dog | Lateral total meniscectomy | Porous polyurethane | - | - | Scaffold implanted group Empty defect |
From 8 to 28 weeks | Implant filled with new meniscal tissue resembling native one. Cartilage degeneration occurred. |
| de Groot JH, Biomaterials. 1996 [38] |
Dog | Total meniscectomy and Longitudinal lesion in the avascular zone |
Aromatic polyurethane (PU) Linear aliphatic PU Aliphatic PU network |
- | - | Aromatic PU group Linear aliphatic PU group Aliphatic PU network group |
For aliphatic PU network (meniscal prosthesis) degeneration of articular cartilage less severe than after meniscectomy. New fibrocartilage generated. |
|
| de Groot JH, Biomaterials. 1997 [39] |
Dog | Full-thickness longitudinal lesion |
50/50 copolymer of L-lactide and r-caprolactone Aliphatic polyurethane |
- | - | copolymer with compression modulus 40 kPa copolymer with compression modulus 100 kPa polyurethane with compression modulus150 kPa |
4 and 26 weeks | Copolymer implants showed better adhesion to meniscal tissue compare to polyurethane. Ingrowth of fibrocartilage for scaffolds with higher compression moduli (100 kPa and 150 kPa). |
| Tienen TG, Biomaterials. 2003 [40] |
Dog | Longitudinal lesion in the avascular part of the meniscus | Porous polyester urethanes based on L-lactide/_-caprolactone |
- | - | Scaffold group Empty defect |
3 and 6 months | Defect repaired in the study group and control group. Scaffold integrated to the host meniscal tissue. Articular cartilage degeneration occurred after scaffold implantation. |
| Tienen TG, Osteoarthritis Cartilage. 2003 [41] |
Dog | Longitudinal lesion created in the avascular part of the meniscus | Porous polyester urethanes based on L-lactide/ caprolactone |
- | - | Scaffold group Empty defect |
3 and 6 months | Articular cartilage degeneration in the polymer scaffold group and empty defect group. |
| Tienen TG, J Biomed Mater Res B Appl Biomater. 2006 [42] |
Dog | Lateral meniscectomy | Estane and PCL-PU scaffolds | - | - | Estane scaffold PCLPU scaffold |
6 months | Meniscus-like tissue regeneration for both implants with tendency towards better results for PCLPU scaffold. |
| Hansen R, J Orthop Res. 2013 [35] |
Dog | 80% resection of the meniscus | Collagen scaffold | - | - | Scaffold group Empty defect |
3 and 6 weeks, 12, 13 and 17 months | Meniscal-like cartilage growth into the collagen scaffold. 4 cases had mild inflammation. 1 case had severe inflammatory response. No evidence of infection. |
| Zhu WH, Mol Med Rep. 2014 [43] | Dog | Medial meniscal lesion | PLA/PGA scaffold | Canine myoblasts transfected with lentivirus expressing hCDMP-2 gene | - | Suture only Suture + scaffold + purified hCDMP-2 Suture + scaffold+ myobolast alone Suture + scaffold + myoblasts expressing hCDMP-2 gene |
3, 8 and 12 weeks | Only group with hCDMP-2 expressing myoblasts produced regenerating tissue, with positive staining for coll I and II and S-100 protein. Faster healing within red-red vs white-white zone. |
Goat Model
The only available trial on the goat model [10] focused on a calcium alginate-based meniscal scaffold augmented with bone marrow mesenchymal stem cells (MSCs). Interestingly, in one of the treatment groups tested by the authors, the bone marrow cells, before being added to the scaffold, were transfected to over-express the insulin-like Growth-Factor 1 (hIGF-1): results were in favor of this genetically manipulated group, since 16 weeks after implantation a complete filling of the meniscal defect and a better histological outcome in cartilage and extra-cellular matrix healing was obtained with respect to the non-transfected BMSCs group, which on the other hand performed better than the scaffold alone group.
Pig Model
The pig model was used to evaluate a vycril mesh scaffold [11], which was tested alone and in association with autologous and allogeneic chondrocytes. Also in this trial a beneficial effect of cellular augmentation was noted: in fact, the best histological outcomes and the larger meniscal healing (12 weeks after implantation) were achieved in the autologous chondrocyte group, followed by the allogeneic chondrocyte group and then by the scaffold alone group.
The pig model was also used to explore the potential of a collagen-based scaffold in a study focusing also on findings obtained in the dog model, thus results will be reported in the following relative paragraph [12].
Sheep Model
Eight papers investigated the results of meniscal scaffolds in the sheep model.
Martinek et al. [13] tested a collagen scaffold with and without autologous chondrocytes (previously cultured in vitro). At 3 months’ histological examination enhanced vascularization, accelerated scaffold re-modeling, higher content of extra-cellular matrix and lower cell number were noted in the pre-seeded menisci in comparison with non-seeded controls.
Chiari et al. [14] used a hyaluronic acid/polycaprolactone meniscal substitute to treat 8 sheep, where the scaffold was used to fill either partial or complete meniscectomies. The implants tested were mechanically stable and showed excellent tissue ingrowth to the capsule. Tissue integration was also observed between the original meniscus and the implant. The histological investigation revealed tissue formation, cellular infiltration and vascularization. After these promising findings, further studies were conducted with the same scaffold. In particular Kon et al. [15] tested the scaffold, in 24 sheep, with or without autologous chondrocyte seeding. In the same trial, also two different fixation techniques were compared: in one case the scaffold was just sutured to the capsule and meniscal ligament, whereas in the other case a trans-tibial fixation of both horns was added. At 6 months’ evaluation, excellent capsular ingrowth was present in all the implants tested; in terms of fibrocartilage formation the addition of autologous chondrocyte proved to be beneficial, whereas better implant appearance and integrity were documented without trans-tibial fixation [15]. In a later study with evaluation performed up to 12 months [16], it was confirmed that seeding the scaffold with autologous chondrocytes increases tissue regeneration capacity, providing a better fibrocartilaginous tissue formation with respect to cell-free scaffold. Authors also revealed that osteoarthritic changes over time were significantly less in the cell-seeded group than in the meniscectomy controls, even though results were not significantly different between cell-seeded and cell-free scaffold [16], thus suggesting that the chondroprotective effect is not directly influenced by cellular addition.
Kelly et al. [17] employed, instead, a hydrogel meniscal implant which was compared to three different treatment groups: meniscal allograft transplantation, empty defect, and sham surgery. Overall results were positive for the hydrogel implant at early follow-up (2 and 4 months) but, at the last evaluation at 12 months, significant cartilage degeneration was observed in the compartment and all the implants presented signs of mechanical failures consisting in complete radial tears located in the posterior third of the implant. The outcome of this hydrogel scaffold was significantly lower than that of the meniscal allograft. Maher et al. [18] reported the results after implanting a porous polyurethane scaffold in a partial lateral meniscectomy model: the evaluations performed 12 months post-op. revealed tissue ingrowth within the scaffold without cartilage damage. However, comparison regarding the status of tibial plateau cartilage did not reveal any significant difference between scaffold implanted and control (untreated) group due to the unexpected tissue self-regeneration occurring in the sheep partial meniscectomy model.
The results of a kevlar-reinforced polycarbonate-urethane scaffold were reported by Zur et al. [19], who found that this kind of biomaterial could provide a chondroprotective effect and reduce cartilage degeneration over time: the authors compared the operated knee with the contralateral healthy one at 3 and 6 months’ follow-up and showed no significant difference in cartilage status between treated and control limbs.
Finally, Gruchemberg et al. [20] tested a silk fibroin scaffold and documented the absence of inflammatory response after implantation, a beneficial effect in preventing cartilage degeneration, and also mechanical features of the regenerated tissue comparable to the native meniscus, even if in three cases mobilization of the scaffold occurred.
Rabbit Model
The first study on the rabbit model dates back to 1992 [21], when a Dacron meniscus prosthesis with polyurethane coating was used. Results of this trial were fairly negative, since poor tissue healing was observed, with low scaffold integration, and the meniscal substitute did not prevent cartilage degeneration with respect to the meniscectomized control group. The same negative outcomes were later reported using two different variants of the aforementioned Dacron meniscal prosthesis: in one case the scaffold was made of Dacron alone (without any coating) [22] whereas in the other one there was the addition of Teflon [23]. In neither cases the scaffolds proved to be beneficial in terms of tissue healing and chondroprotection, thus sentencing the ineffectiveness of this particular construct for meniscal regeneration.
In more recent times new biomaterials have been tested with more encouraging results. Testa Pezzin et al. [24] developed a Poly(p-dioxanone)/poly(L-lactic) acid scaffold which provided good mechanical stability after implantation and showed to stimulate tissue regeneration. Similarly, the group led by Kobayashy has published 3 papers where they tested a polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus which exhibited enough mechanical resistance without dislocation or breakages and showed a chondroprotective effect in the lateral compartment where it was implanted. The authors evaluated the outcome of this meniscal scaffold implantation up to 2 years, documenting lower OA progression in the scaffold group with respect to empty controls [25-27].
Angele et al. tested a hyaluronan/gelatin composite scaffold with and without pre-cultured bone marrow-derived MSCs: at 12 weeks of follow-up the best results were obtained in the cell-augmented group, where meniscus-like fibrocartilage was documented and also a greater cross-sectional width of the scaffolds with respect to cell free implants, which produced mainly fibrous repair tissue [28].
In 2010 Zellner et al. [29] evaluated the use of cell augmentation to a hyaluronan/collagen meniscal scaffold tested in the rabbit model. In this trial 5 different treatment groups were compared: empty defect, scaffold + bone marrow concentrate (BMC), scaffold + platelet-rich plasma (PRP), scaffold + pre-cultured chondrogenic MSCs, scaffold + non precultured MSCs. Furthermore, the scaffold alone was used as a control in a meniscal defect created in the contra-lateral knee of all rabbits. At 12 weeks the authors documented that PRP and BMC did not improve tissue healing with respect to the scaffold alone. Histological results after using the scaffold + BMC or PRP were modest, revealing just fibrous healing in the implanted area. When looking at the scaffold + pre-cultured MSCs, a fibrocartilage-like repair was achieved with a partial integration within the native tissue. The best results were instead obtained by the scaffold + non pre-cultured MSCs, where the integration was complete and the histological aspect more similar to the native tissue [29]. The same authors, in a later similar study [30], confirmed these results, thus proving that MSCs play a crucial role in promoting a superior meniscal regeneration, at least when used together with the particular hyaluronan/collagen scaffold tested in this experimental setting [30].
A poly-glycolic acid based scaffold with the addition of expanded allogeneic meniscal cells has been used by Kang et al. as a replacement after medial meniscectomy [31]. The study, despite not having any cell-free control group, demonstrated that regenerated neomenisci were similar to normal meniscal cartilage in both gross and histological appearance. Esposito et al. tested a porous poly(L-co-D,L-lactic acid)/poly(caprolactone-triol) scaffold with and without the addition of fibrochondrocytes [32], which were pre-seeded onto the scaffold and expanded in vitro for 21 days. A good integration of the implants was observed, without signs of foreign body reaction, and better results in tissue regeneration were documented for the cell-seeded scaffold.
The most recent study on the rabbit model was published by Oda et al. [33], who tested a type I collagen scaffold with or without augmentation by infra-patellar fat pad, which is a source of MSCs. Eight week after the procedure the rabbits were sacrificed and the best meniscal regeneration (evaluated through the Ishida Score) was documented in the fat pad group, which also revealed a superior expression of type I and type II collagen at immuno-histochemical essays. Further in vivo analysis proved that the addition of infra-patellar fat pad was able to reduce the secretion of metalloproteinases and other interleukins that could impair tissue regeneration.
Dog Model
The first attempt to replace meniscal tissue was performed in 1983 by Toyonaga et al. who implanted a Teflon scaffold [34] and published the first study showing the feasibility and the potential of this novel regenerative approach. Almost a decade later, in 1992 Stone et al. [12] used a collagen based scaffold revealing promising results in terms of meniscal fibrocartilage regrowth in the dog model, although the same biomaterial proved to be less effective in the pig model tested by the same authors. More recently, a study authored by Hansen et al. [35] investigated the potential of a collagen-based scaffold: despite a documented well integration of the scaffolds, the authors reported a significant occurrence of inflammatory response after implantation, thus suggesting the necessity for further improvements of this construct.
In the following years an increasing interest emerged toward another biomaterial for tissue regeneration: polyurethane. Several studies have been published on this specific material: the first reports have been authored by the group of Klompmaker [36, 37], who documented the biocompatibility of this scaffold together with its capability of stimulating the formation of new meniscal fibrocartilage with good mechanical properties. DeGroot et al. [38] obtained good results using an aliphatic polyurethane network-organized scaffold and further studies by the same authors proved that regenerative properties of polyurethane could be improved by a chemical augmentation, using a copolymer of L-lactide and r-caprolactone aliphatic polyurethane [39]. This novel formulation allowed for a better adherence to the native tissue and was responsible for a superior tissue regeneration. Further developments were proposed and tested by Tienen et al. who realized a scaffold made of porous polyester urethanes based on l-lactide/e-caprolactone [40-42]. This meniscal substitute achieved interesting results in terms of histological and biomechanical parameters [40, 41] and, when compared to an estane-based scaffold [42], it provided slightly superior results, thus leading to its application in the clinical practice with good results as demonstrated by some human trials [7, 8]. The most recent study on the dog was published by Zhu et al. [43] who performed a complex experiment, whose principal aim was to investigate the role of hCMP-2 (Human Cartilage-derived Morphogenetic Protein 2) in meniscal repair. To this purpose, canine myoblasts were transfected with lentiviruses carrying the hCMP-2 gene. These myoblasts were then loaded onto a PLA/PGA scaffolds and the whole construct placed onto the meniscal defect site. Authors found that purified canine myoblasts expressing the hCDMP-2 gene were able to promote meniscal fibrocartilage healing: the tissue in the red-red zone was regenerated more rapidly than that in the white-white zone. Furthermore, the results obtained with this particular implant were superior to those achieved by using canine myoblasts or recombinant hCMD-2 alone.
DISCUSSION
The implantation of biomaterials to stimulate the replacement of injured meniscal tissue represents a fascinating option for clinicians, since even partial damage or removal of the meniscus have been demonstrated to alter the joint biomechanics, leading to the early onset of OA [3]. The use of meniscal scaffolds aims at giving a structural contribute for defect repair and at stimulating the healing processes of damaged tissues, in order to provide a reparative tissue that resembles the properties of the native one [5]. Our search identified an increasing number of preclinical papers dealing with scaffold-based meniscal replacement in the animal model. Various biomaterials have been investigated at preclinical level, with the aim of finding the ideal one, in terms of biocompatibility and regenerative potential, to be translated to the clinical use. Beside some negative results and some controversial findings, the overall results of the studies analyzed confirmed the potential of this approach with promising findings in terms of tissue regeneration and in some studies even a chondro-protective effect provided by the implanted biomaterial.
The systematic analysis of the available literature underlined that most papers evaluated meniscal replacement in the animal model with cell-free scaffolds. While poor results have been obtained by preliminary attempts and despite the ineffectiveness of some biomaterials, progresses made by biomaterials research in the last years have yielded to the development of a new generation of scaffolds able to stimulate the intrinsic tissue regeneration ability [44]. These cell-free scaffolds provided good results while avoiding the risks related to cell manipulation [44] (e.g. bacterial contamination and phenotype loss,…) and the regulatory limitations which may hinder a subsequent translation in the clinical practice. In this direction, various biomaterials have been developed and tested through preclinical research, with the challenge of guiding the regeneration of a tissue resembling the specifics of the native one. In general, promising results have been shown in the preclinical setting in terms of morphology and function, even though many differences emerged with respect to the healthy tissue in terms of mechanical and biochemical analysis. Beside a few constructs showing limited biocompatibility [35] or ineffectiveness in terms of chondroprotection and tissue healing [19, 21, 22], most of the biomaterials evaluated confirmed the potential of this surgical approach for the treatment of meniscal loss, with satisfactory integration of the implant and tissue regeneration. Among them, polyurethane is the most studied, with overall promising results in terms of biocompatibility and chondroprotective effect [36-42]. After these good histological and biomechanical findings, polyurethane-based meniscal substitutes are currently applied in the clinical practice, with satisfactory results at short- to mid- term follow-up [7, 8]. However, no comparative studies have been performed to prove the superiority of one biomaterial to others, and new treatments are currently being developed aiming at further improving the results obtained by this regenerative approach to treat and restore meniscal tissue damage.
Regenerative treatments are a complex and rapidly evolving field of medical research, where an increasing number of papers shows similar good results for many different products. Moreover, beside the literature limitations which do not allow to clearly prove superiority of one procedure to the others, several not scientific factors also influence the research direction, such as economic and regulatory aspects [46]. In this scenario, cells occupy a controversial role: in fact, despite the good results suggested for cell augmentation, currently preclinical research is focusing more on the evaluation of cell-free constructs [45], and cell-free techniques are the only ones translated into the clinical practice, where no trials about cell-augmented techniques have been reported yet. The use of cell-free scaffolds allows to avoid the regulatory obstacles and the costs related to cell manipulation, and also the more complex organization needed to offer a cell-based treatment [44, 47], but some animal trials suggested that cell-augmentation might provide superior regenerative potential in terms of tissue quality. However, the combination of scaffolds and cells has not been extensively investigated and only a few comparative studies are currently available, so it is not possible to draw definitive conclusions on the real effectiveness of cell augmentation. Moreover, the real clinical benefit remains unknown, both in terms of function improvement and joint preservation, and further research is needed in this direction. The analysis of the cell sources also showed no sufficient evidence, and the available evidence doesn’t provide clear indications for the most suitable one. MSCs have been increasingly used because of their characteristics, which would suggest them as the ideal source for regenerative procedures due to their properties of self-renewal and stemness maintenance, their potential for differentiation into cells forming multiple mesodermal tissues, other than their trophic and immuno-modulatory effects [48]. On the other hand, other studies applied cultured meniscal cells, chondrocytes or fibrochondrocytes, and overall good results have been reported also when using differentiated cells. Regardless of the limits of the literature, the controversial findings, and the need for further studies, it is important to underline that among the studies reporting on cell-augmented scaffolds, in general, superior results were reported adding cells compared to scaffold alone or even to PRP augmented implants. However, these papers present with a marked heterogeneity in terms of different cell sources, dosage, scaffold materials, animal models, and defect types, thus making difficult to draw a conclusion on the real effect of cells on the scaffold-based approach. Beyond cellular augmentation, also novel bio-engineered strategies are currently under investigation in the attempt of improving tissue regeneration: a few recent studies tested the use of growth factors (such as IGF-1 and hCMP-2) through cellular transfection using viral vectors [10, 43]. The results reported seem to be quite encouraging even if the very low number of trials and the large variety of molecules that could be potentially used warrant further research in this particular field, remembering that such approaches are extremely expensive and, at least for the moment, their translational potential is limited also by ethical and regulatory issues.
Among the literature limitations, beside the heterogeneity of the biomaterials, cell sources and processing methods, and the lack of comparative studies which prevents to clearly identify which techniques are more effective, other aspects should be considered since they may influence the outcomes of the regenerative procedures evaluated in these studies. First, the choice of the experimental model is key for the success or failure of each construct, with results varying according to the type of animal evaluated, and also the type of lesion, medial or lateral, partial or complete, acute or chronic etc… may influence the results documented on the potential of the studied scaffolds.
When looking at clinical practice, it is important to remember that scaffolds are used mainly in chronic meniscal lesions rather than in acute lesions (which is instead the classical setting of animal trials): sometimes scaffolds are implanted many years after meniscectomy and the articular environment could play a key role in determining the success of meniscal regeneration. Furthermore, meniscal scaffolds in clinical practice are used to treat just partial meniscal defects, whereas in the animal model scaffolds are often used as total meniscal replacement: it is still unclear, in the human setting, if a critical size exists beyond which the mechanical stability and/or the regenerative potential of scaffolds is impaired. Moreover, the preclinical level gives important indications for the development of new treatments, but it doesn’t completely mirror the human setting. For example, collagen scaffolds that have shown controversial results in the animal model [12, 35], have been extensively used on humans with satisfactory outcomes [42], and on the other hand it is also possible that biomaterial suggesting good results in the animal model may not be as successful after reaching the clinical practice.
In conclusion, the present review underlined the potential of biomaterials’ implantation in terms of meniscus regeneration and chondral protection, but at the same time it highlighted the difficulties in understanding the most suitable scaffold and surgical approach, due to the heterogeneity of products used and preclinical models, as well as the design of the published studies. The literature analysis highlighted that biomaterial properties, such as biocompatibility, are key for the success of such regenerative procedures, which may provide beneficial effects in the treated joints, and limited but consistent evidence suggests that cell-augmentation may be beneficial for scaffold-based meniscal tissue regeneration. Nonetheless, clinical research is currently focusing on cell-free procedures, due to several reasons including scientific, economic, and regulatory aspects, and good results have been obtained in humans treated with these meniscal scaffolds. The overall preclinical evidence supports the use of scaffolds to restore meniscal tissue damage, and progresses in biomaterial properties may further improve the results obtained by the currently available scaffolds. High quality studies are needed to prove the benefit of each technique by directly comparing different strategies, increasing the knowledge on scaffold-based treatments and the best ways for exploiting the self-regenerative potential of the body. Among the most promising strategies to improve the final outcome, cell augmentation deserves particular attention. The available preclinical evidence, despite being not conclusive, suggests that cells may enhance tissue regeneration with respect to the use of biomaterials alone, which warrants further research in this direction. Exploring the best cell sources, manipulation and application modalities could contribute to a possible translation of bioengineered tissues in the clinical practice, being aware, however, that the beneficial effects of cellular augmentation reported in animal trials might be not confirmed when used in the human model.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The present manuscript was partially supported by “European Project BIOINSPIRE- Training program on new bio-inspired bone regeneration (FP7-PEOPLE-2013 ITN-607051)”.


