All published articles of this journal are available on ScienceDirect.
Lower Dislocation Rate Following Total Hip Arthroplasty via Direct Anterior Approach than via Posterior Approach: Five-Year-Average Follow-Up Results
Abstract
Objective:
The aim of the study was to compare the dislocation rate between total hip arthroplasty (THA) via direct anterior approach (DAA) and via posterior approach (PA).
Methods:
We compared a consecutive series of 139 THAs via DAA with 177 THAs via PA. All study patients received ceramic-on-ceramic bearing surfaces and similar uncemented prostheses. Dislocation-free survival after THA was estimated using the Kaplan–Meier survival method and compared between groups using the log-rank test.
Results:
In the DAA group, none of 139 hips experienced dislocations in five-year-average follow-up. In the PA group, seven hips experienced dislocations among 177 hips (4 %). The dislocation was significantly less in the DAA group compared to the PA group (p = 0.033).
Conclusion:
The dislocation rate of THA via DAA was significantly less than that of THA via PA.
BACKGROUND
Dislocation remains a common complication following total hip arthroplasty (THA) [1-3]. The causes of dislocation are multifactorial [4], and some studies have revealed that the surgical approach could affect the rate of dislocation [4, 5].
Direct anterior approach (DAA) does not require a detachment of muscle or tendon to perform THA [6-15]. Recently, DAA has attracted a great deal of interest because preserving muscle attachments to bone may improve hip stability [6-9]. Studies revealed dislocation rates of 0%–1.5% in THA via DAA [10-13]. Although THA via DAA is believed to reduce the dislocation rate following THA, no comparative study has proven that the incidence of dislocation is lower than any other surgical approach [13-15].
We compared a consecutive series of 139 THAs via DAA with 177 THAs via posterior approach (PA) with emphasis on dislocation rate. Two hypotheses were tested: (1) the dislocation rate of THA via DAA is lower than via PA and (2) the survival rate as revision for any reasons does not differ between the two surgical approaches.
METHODS
We reviewed the clinical records and X-rays of all subjects after this study were approved by the ethics committee of our institute with the register number 2013N09020.
The inclusion criterion for this study was that the patients had undergone THA via DAA performed by a single surgeon between February 2006 and March 2009. The exclusion criterion was the patients who had undergone THA via any other surgical approach during the study period. Between February 2006 and March 2009, 149 THAs were performed by the single surgeon. Ten THAs were excluded because the THAs were performed via PA in order to graft a bulk femoral head for acetabular bone deficit. Thus 139 THAs via DAA were included in the study, and were designated as the DAA group. All bearing surfaces of studied patients were third generation ceramic-on-ceramic (Biolox Forte, Ceramtec, Plochingen, Germany). We used 28-mm-diameter head in 85 hips, and 32-mm-diameter head in 54 hips. In all THAs, a hemispherical plasma-sprayed titanium shell (Plasmacup; B. Braun Aesculap, Tuttlingen, Germany) and a slightly tapered, rectangular, collarless titanium alloy stem with fins for increased rotational resistance (BiCONTACT; B. Braun Aesculap) were implanted without cement. Sixty-nine of the THAs in the DAA group were included in our previous study [16].
One hundred and seventy-seven THAs via PA using Biolox Forte, Plasmacup, and BiCONTACT between January 2000 and January 2006 comprised the PA group which served as the control group in the study. All THAs via PA were performed or supervised by the same surgeon who performed THAs via DAA in this study. A total of 5 surgeons performed THA via PA. In PA group, all hips were received 28-mm-diameter head. One hundred and twenty-four of the THAs in this group were included in our previous case series [17].
Surgical Technique and Postoperative Treatment
The procedure used in the DAA groups during the study period followed the surgical technique described by Nakata et al. and Oinuma et al. [13, 18]. A standard operative table was used. After retracting the tensor fasciae lata and sartorius, the anterior aspect of the hip capsule was exposed by displacing the gluteus minimus and the rectus femoris muscle. The anterior aspect of the hip capsule was routinely removed. To expose the proximal femur and elevate it almost to the skin level to allow access to the femoral canal, the superior capsule was released [19].
In the PA group, the posterior joint capsule was removed, and the incised short external rotators were reattached to the greater trochanter.
In both groups, antibiotic prophylaxis, with a first-generation cephalosporin, was intravenously administered perioperatively and then every eight hour for the first 48 hour postoperatively. Physiotherapy with full weight bearing was allowed one day after postoperatively. Although no brace was used for the patients in the DAA group to prevent dislocation, an abduction brace was used for the patients in the PA group when surgeon deemed it necessary.
Outcome Measurements
The primary outcome of the comparative study was postoperative dislocation. We used the Kaplan-Meier method to estimate the cumulative probabilities of postoperative dislocation. The survivorship curves with dislocation as the end-point were compared between the groups using the log-rank test. The chi-squared test was also applied to compare the dislocation rate.
In addition, implant survival rates with revision surgery, irrespective of the reason, as the endpoint were compared. Any complication which occurred prior to the patients’ final follow-up was reviewed.
The angles of inclination and anteversion of acetabular shell were determined on immediate postoperative anteroposterior X-rays in radiographic definition [20]. All radiographs were measured by the same observer. The inclination and anteversion were measured with computer software (computer-assisted measurement of total hip arthroplasty, Japan Medical Material, Osaka, Japan). Using the computer software, an ellipse was fitted to the rim of acetabular shell on X-ray. Inclination was measured directly, while anteversion was calculated using the ratio between the lengths of the minor and major axes of the ellipse (Fig. 1) [21, 22]. The rate of outlier was compared between groups when safe range was defined as 30 to 50 degree in inclination and 5 to 25 degree in anteversion, respectively [21].
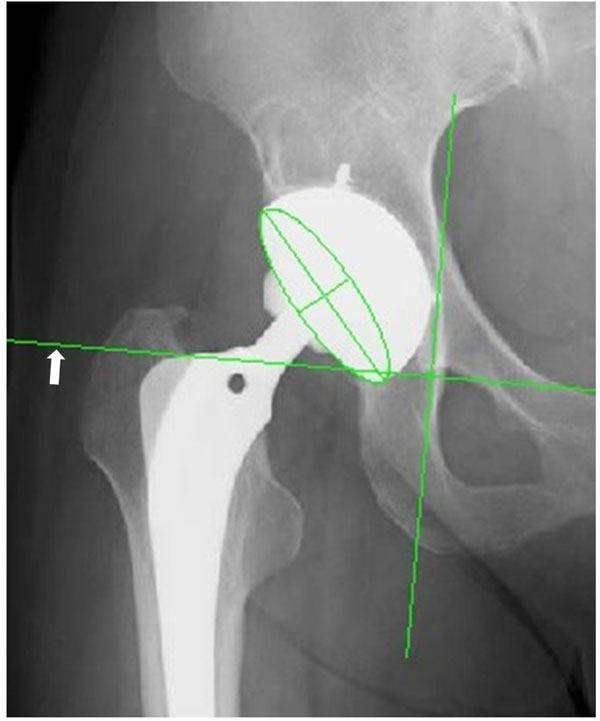
Determination of acetabular cup inclination and anteversion using computer software (computer-assisted measurement of total hip arthroplasty, Japan Medical Material, Osaka, Japan). The white arrow shows tear drop line.

Kaplan–Meier curves of the survival rate with postoperative dislocation as the end-point. The dislocation rate was significantly low in the DAA group compared with that in the PA group (log-rank p = 0.033).
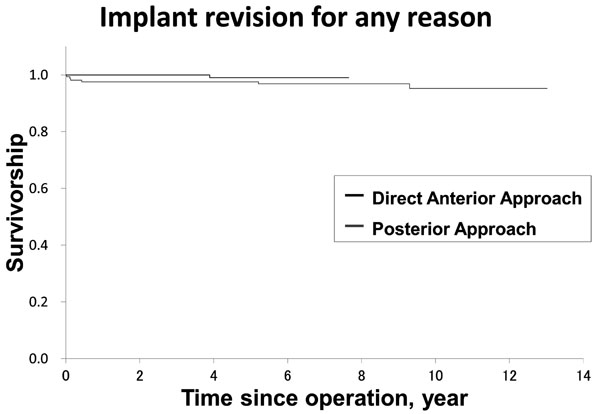
Kaplan–Meier curves of the survival rate with revision surgery as the end-point. There were no significant differences in survivorship (log-rank p = 0.28).
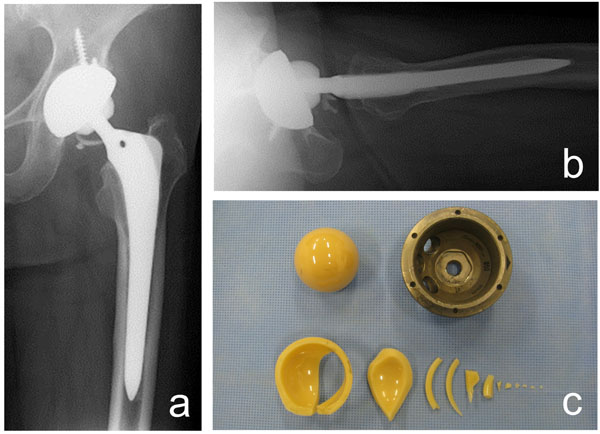
Ceramic liner fracture in a 61-year-old woman. The ceramic fracture occurred 3.8 years after a left total hip arthroplasty via the direct anterior approach. Fragments of the fractured ceramic liner can be seen on the anteroposterior (a) and lateral X-ray (b). The photograph shows the retrieved ceramic liner, ceramic head, and acetabular shell (c).
The stability of acetabular component and femoral components and presence of osteolysis were determined from X-rays at final follow-up. Loosening of acetabular component was defined as a migration of 2 mm or a change in the abduction angle of that component of 5 degree [22]. A subsidence in the vertical distance of > 4 mm or a change in the varus angle of >2 degree was considered to indicate stem migration and loosening [22]. Osteolysis was defined as localized bone loss with distinct borders that could be outlined with a pencil and were not apparent on the baseline X-ray [23].
Statistical Analysis
To compare patient background characteristics, we used the student’s t and chi-square tests for continuous variables and nominal scales, respectively. In addition, we used the Kaplan–Meier method to compare the dislocation and implant survival rates, and the log rank test to compare the differences between the groups. The rate of complications including dislocation was compared with chi-square test. Differences in variance of inclination and anteversion of acetabular shell between the groups were analyzed using the chi-square test. P < 0.05 was considered statistically significant.
RESULTS
The demographics and diagnostic data of the patients are outlined in Table 1.
Patient demographics and baseline clinical characteristics.
| Direct Anterior Approach (n = 139) | Posterior Approach (n = 177) | P-Value | |
|---|---|---|---|
| Age, years | 66.7 ± 9.8 | 61.7 ± 10.3 | <0.0001* |
| Sex (F/M) | 125/14 | 147/30 | 0.08† |
| Height, cm | 152.3 ± 7.8 | 153.8 ± 8.3 | 0.14* |
| Weight, kg | 53.6 ± 9.0 | 56.5 ± 8.7 | 0.01* |
| Body mass index, kg/m2 | 23.0 ± 3.0 | 23.9 ± 3.7 | 0.03* |
| Preoperative diagnosis (OA/ON/RA) | 127/7/5 | 153/20/4 | 0.12† |
| Preoperative d'Aubigne and Postel hip score, points | 10.6 ± 1.4 | 11.3 ± 1.8 | 0.0003* |
| Follow-up period, years | 5.3 ± 1.2 | 9.2 ± 2.5 | <0.0001* |
Results are expressed as mean ± standard deviation, unless stated otherwise.
OA, osteoarthritis; ON, osteonecrosis; RA, rheumatoid arthritis
* P-values were determined using Student’s t-test
† P-values were determined using the χ2 test
Comparison of postoperative complications between the two approaches.
| Direct Anterior Approach (n = 139) | Posterior Approach (n = 177) | P-Value† | |
|---|---|---|---|
| Dislocation | 0 | 7 | 0.018 † |
| Surgical site infection | 0 | 2 | 0.21 † |
| Femoral perforation | 2 | 0 | 0.11 † |
| Intraoperative fracture of greater trochanter | 2 | 0 | 0.11 † |
| Transient femoral nerve palsy | 1 | 0 | 0.26 † |
| Transient sciatic nerve palsy | 0 | 2 | 0.21 † |
| Ceramic line fracture | 1 | 1 | 0.86 † |
| Clicking sound | 3 | 11 | 0.082 † |
| Squeaking sound | 2 | 0 | 0.11 † |
† P-values were determined using the χ2 test.
In the DAA group, no patients experienced dislocation by the final follow-up. In the PA group, anterior dislocation occurred in three hips and posterior dislocations in four. The seven dislocations occurred at 1, 2, 5 weeks, 3 months, 2.5 and 5.2 years after surgery. Three of 7 patients who experienced dislocation wore the abduction brace. The dislocation rate was significantly low in the DAA group compared with that in the PA group (log-rank p = 0.033; Fig. 2). The significance of the difference was also confirmed by chi-squared test (p = 0.018).
The survivorships with the end-point as revision are shown in Fig (3). In the DAA group, one patient (one hip) was revised due to ceramic liner fracture at 3.8 years after surgery (Fig. 4). In the PA group, six patients (six hips; one hip each) underwent component revisions. Three patients had revision surgeries for dislocation at 1, 6 weeks and 5.2 years after surgery. Two with deep infection underwent revision surgeries at 1 and 5 months after surgery. One was revised due to ceramic liner fracture 9.3 years after surgery. There were no significant differences in survivorship when revision was used as the end-point (log-rank p = 0.28).
All complications are outlined in Table 2. There were no significant differences in the overall complication rate between the two groups (11/139 hips in the DAA group versus 23/177 hips in the PA group; p = 0.14).
In the DAA group, the average cup inclination and anteversion angles were 44.8° ± 3.8° and 18.5° ± 4.2°, respectively, whereas in the PA group, they were 47.2° ± 6.2° and 18.0° ± 6.0°, respectively. More hips were outside the safe range in the PA group (63 of 177 hips, 34.5 %) than in the DAA group (18 of 139 hips, 12.9 %) (p < 0.0001, chi-square test). Of the 7 hips that experienced dislocation in PA group, the acetabular shell was outside the Lewinnek’s safe range in 3 hips. All of these 3 hips dislocated anteriorly. Remaining 4 hips were inside the safe range, and experienced posterior dislocation.
All acetabular and femoral components were stable at final follow-up. No radiographic evidence of osteolysis was observed.
DISCUSSION
Most importantly, this study demonstrated that the dislocation rate of THA via DAA was significantly lower than THA via PA.
Many case series involving more than 1,000 THAs via DAA have shown low dislocation rates. Bhandari et al. conducted a multi-centre observational study of THA via DAA, and found that eight of 1,277 hips (0.6%) suffered dislocation [10]. In a study by Sariali et al., 27 of 1,764 hips (1.5%) suffered dislocation [11]. And, Siguier et al. reported dislocation in 10 of 1,037 hips (1.0%) [12]. Although these studies had the limitation of having no control group, their findings suggested that THA via DAA had a low dislocation rate.
Nakata et al. conducted a retrospective comparison between a consecutive series of THA via PA during one period and a consecutive series of THA via DAA during the subsequent period [13]. In their study, none of 99 patients in the latter group experienced dislocation, whereas one of the 96 patients in the former experienced dislocation. The dislocation rate was not significantly different between the groups. One explanation for the result might be the small sample size. Because the dislocation rate after THA in the study reported by Nakata et al. was extremely good in comparison with previous studies [24], more patients should be included in further studies to investigate differences in the dislocation rate between groups.
Soft tissue tension is one of the factors which predict postoperative dislocation [24, 25]. DAA does not detach muscle or tendon during THA [12], thereby maintaining soft tissue tension. This advantage of DAA could explain the lower dislocation rate in our study.
A number of limitations must be noted in this study. The difference in the period during which the two surgical approaches were performed is the most important limitation [26]. Although studies using a design similar to our study have frequently been used to compare the clinical results of different surgical approaches for THA [13, 27], the lack of a control group operated on during the same study period could over-estimate the results by regression to the mean [28].
All 177 hips in the PA group received 28-mm-diameter head, whereas 85 of 139 hips received 28-mm-diameter head and remaining 54 hips received 32-mm-diameter head in the DAA group. Larger head provides a more stable articulation, and might achieve lower dislocation rate [4]. In addition to the head diameter, there were significant differences in patients’ demographic including age, weight, body mass index, preoperative d'Aubigne and Postel hip score, and the number of surgeon to perform THA between groups. And, only PA group wore abduction brace for preventing the dislocation. These differences could bias the results of our study although there were not many studies supporting that these factors significantly affect the dislocation rate [29-32].
Acknowledging these limitations, we believe that our result provides useful information for surgeons as to their choice of surgical approach in THA.
CONCLUSION
The dislocation rate of THA via DAA was lower than THA via PA. DAA might be preferable to PA to reduce the dislocation.


