All published articles of this journal are available on ScienceDirect.
In Vitro Elution Characteristics of Gentamicin and Vancomycin from Synthetic Bone Graft Substitutes
Abstract
Objects:
Beta tricalciumphosphate pellets loaded with individualized antibiotics may represent novel options in the treatment of osteomyelitis and infectious bone disease. Here, the in vitro antibiotic elution of vancomycin and gentamicin from the synthetic bone graft substitutes Cerasorb® and Cerasorb M® was tested.
Methods:
Antibiotic elution and concentration of gentamcin and vancomycin were measured using photometrically-based measurement and homogeneous particle-enhanced turbidimetric inhibition immunoassays (PETINIA).
Results:
Initially both materials showed a high release of the loaded antibiotics, with Cerasorb M® showing lower release levels for gentamicin and vancomycin than Cerasorb®. Gentamicin concentrations of Cerasorb M granules and Cerasorb were below the minimum detectiontreshold until day four and six of the experiment respectively. The vancomycin release-level followed a similar pattern, although the vancomycin concentration eluted by Cerasorb M® granules stayed above the detection threshold during the experimental time.
Conclusions:
Cerasorb® and Cersorb M® may represent a new treatment option in osteomyelitis and infectious bone disease.
1. INTRODUCTION
The treatment of osteomyelitis remains a challenging problem for orthopaedic surgeons. Reaching a chronic state of osteomyelitis, the local vascularity is compromised. Therefore obtaining an effective local antibiotic concentration is hardly gained through oral or parental administration. Based on this knowledge, the local application of antibiotics, besides surgical debridement, represents a viable alternative treatment to handle osteomyelitis by reaching higher local antibiotic concentrations and minimizing systemic side effects [1].
Since the introduction of antibiotic-impregnated composite and chains, the local application of antibiotics is a common and widely used procedure in the fields of trauma and orthopaedic surgery [2, 3].
The main disadvantages in the utilisation of antibiotic-impregnated chains are the necessity of a second surgery to remove the material, as well as not being able to provide individualized chemotherapy in terms of local administration of different antibiotics.
Today there are several bone-graft substitutes available, which can be used as a controlled release delivery system for antibiotics. E.g. the usage of calcium sulfate as a bone graft substitute for bone lesions is known for a long time, with proven efficiency in experimental and clinical trials [4-7]. Adversely several trials showed a transient cytotoxic effect of calcium sulphate, resulting in inflammatory reactions [8-10].
Nevertheless in several trials calcium sulphate was used as an antibiotic-carrier material and has proven its efficiency as well as its security as a carrier substance [11, 12].
The major drawback in earlier studies of calcium sulphate as an antibiotic-carrier material was the addition of the appropriate antibiotic to the calcium sulphate before hardening, creating possible risks of impairing the antibiotic activity due to hardening or sterilization procedures [11].
Today there is a formulation of the material solving this problem by loading the antibiotic after sterilization, directly before application [13].
Due to the cytotoxic effect of pure calcium sulphate and the probable loss of antibiotic efficiency during hardening, several combinations of calcium sulphate with different materials were developed, e.g. the combination of calcium sulphate and nanochrystalline hydroxyapatite. This combination is available in different moulds, which can be loaded with antibiotics after sterilization or hardening. Over a period of 10 days the composite material of calcium sulphate and hydroxyapatite showed a nearly complete release of the loaded antibiotics. Within these 10 days of trial the composite material revealed high initial antibiotic release with subsequent decline. The different release of antibiotics between the combination of a calcium sulphate/hydroxyapatite mixture and pure calcium sulphate was compared in several studies. Such studies found a higher early release (during the first 5 days) for the calcium sulphate/hydroxyapatite mixture. After 5 days of trial pure calcium sulphate showed a higher antibiotic release [14].
The same studies also showed an excellent resorption rate, biocompatibility and antibiotic release for the calcium sulphate/hydroxyapatite composite material.
Several other materials were shown to display similarly high levels of biocompatibility, resorption rate and osseointegration. Widely available are calciumphosphates, mainly hydoxyapatite and tricalciumphosphate. Both materials are reasonable alternatives for the current gold standard in the treatment of bone lesions, the autogenic cancelous bone repair [15, 16].
The aim of this study was to evaluate the applicability of Cerasorb® (Curasan AG), a beta tricalciumphosphate ceramic, as a carrier substance for antibiotics by measuring in vitro the release kinetics of gentamicin and vancomycin [17, 18].
Former studies showed the principal usage of beta- tricalciumphosphate as a carrier substance for proteins like BMP-2 [19].
2. MATERIALS AND METHODS
2.1. Antibiotic Carrier
We used Cerasorb® and Cerasorb M® as it was supplied to us by the manufacturer Curasan AG, Germany. Cerasorb® ortho has an open interconnecting microporosity of 35 % and is available in a round shape. Cerasorb M® features an interconnecting, open multi porosity with micro, meso and macro pores (5 µm - 500 µm) and a total porosity of approx. 65%. The granules are polygonal, i.e. irregularly shaped, and facilitate canting and interlocking in the defect cavity. Micro movements are largely prevented.
Both materials have a phase purity of more than 99% ß-tricalciumphosphate. For this study we had 10 pieces of Cerasorb® and Cerasorb M® at our disposal. Each of which measured 1x1x1 cm. One granule of each group was tested on the absorption of the antibiotics vancomycin and gentamicin. The remaining granules were tested on the release kinetic either of vancomycin or gentamicin, 4 granules per each antibiotic.
2.2. Antibiotic Uptake and Release
Both bone graft substitutes were loaded with antibiotics via a soaking process. 5 pellets of Cerasorb® and Cerasorb M® were soaked for 1 minute in a gentamicin (Gentamicin-ratiopharm 80 SF, Ratiopharm GmbH, Ulm) solution (40 mg/ml). The pellets were kept at room temperature and completely covert by the solution during the soaking process.
The vancomycin solution was produced by injecting 10 ml water for injection into the dry matter vancomycin (Vancomycin CP, Hikma Pharma GmbH, Gräfeling), thus creating a solution of 50 mg/ml. 5 pellets of Cerasorb® and Cerasorb M® were soaked for 1 minute in the vancomycin solution. As above the pellets were completely covered by the solution and kept at room temperature during the soaking process.
To measure the antibiotic uptake one pellet of Cerasorb® and Cerasorb M® loaded with either one of the antibiotics was pestled. Subsequently the material was mixed with 4 ml PBS and incubated at 37° for 24 hours. Supernatants were removed and stored at -80°. The antibiotic content was measured in the supernatant.
In order to determinine the release kinetics each of the two antibiotics was soaked into 4 pellets of Cerasorb® and 4 pellets of Cerasorb M®. We examined the elution in 4 ml phosphate-buffered saline (PBS) at pH 7,4 and 37°. Every 24 hours the supernatant was taken from the solution and 1 ml was preserved at -80°. The remaining pellets were cleaned with PBS and again covered with 4 ml of fresh PBS. This procedure was continued for 10 days.
The taken samples were preserved at -80° and defrosted immediately before being assayed with either MULTIGENT Gentamicin or Vancomycin assay (Abbott Laboratories Inc., USA), both homogeneous particle-enhanced turbidimetric inhibition immunoassays (PETINIA). Both assays were based on competition between drug in the sample and drug coated onto a microparticle for antibody binding sites of the gentamicin or vancomycin antibody reagent. The genta-micin- or vancomycin-coated microparticle reagent was rapidly agglutinated in the presence of the anti-genta-micin/vancomycin antibody reagent and in the absence of any competing drug in the sample.
The rate of absorbance change was measured photo-metrically and was directly proportional to the rate of agglutination of the particles. When a sample containing gentamicin/vancomycin was added, the agglutination reaction was partially inhibited, slowing down the rate of absorbance change. A concentration-dependent classic agglutination inhibition curve can be obtained with maximum rate of agglutination at the lowest gentamicin/vancomycin concentration, and the lowest agglutination rate at the highest gentamicin/vancomycin concentration.
The antibiotic concentration of the eluates was determined by an automatic antibiotic concentration analysing system (Architect 4000, Abbott Laboratories Inc., USA).
3. RESULTS
The loading of the granules showed no technical difficulties and does not affect the macroscopic or mechanical features of the granules after being loaded.
Antibiotic uptake of gentamicin was 406,38 μg/ml for Cerasorb ® and 145,2 μg /ml for Cerasorb M®. The uptake of vancomycin was 1552,4 μg /ml for Cerasorb®, Cerasorb M ® granules had an uptake of 149 μg/ml.
Release kinetics of both granule types were measured as described by Rauschmann et al. [14]. Initially both materials showed a high release of the loaded antibiotics, Cerasorb M® showing a lower initial release level for gentamicin and vancomycin than Cerasorb®. At day 4 the gentamicin concentration of the Cerasorb M® granules was under the detection treshold, for Cerasorb® the gentamicin concentration was undetectable at day 6 (Fig. 1, Table 1).
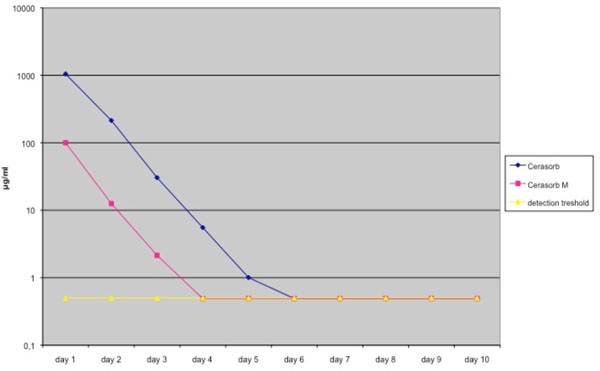
Elution pharmacokinetics of Gentamicin from Cerasorb® and Cerasorb M®. Antibiotic release is expressed in μg /ml. Mean values were derived from a total of 4 different tests. Granules were loaded in a solution of 40 g/L antibiotics.
Elution Pharmacokinetics and Standard Deviation of Gentamicin from Cerasorb® and Cerasorb M®. Antibiotic Release is Expressed in µg /ml. Mean Values were Derived from a Total of 4 Different Tests. Granules were Loaded in a Solution of 40 g/L Antibiotics
| Elution Kinetics of Gentamicin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | |
| Cerasorb | 1046.4675 | 214.3825 | 30.475 | 5.5325 | 1.005 | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 |
| Standard Deviation | 421.3 | 18.8 | 3.4 | 0.61 | 0.26 | 0 | 0 | 0 | 0 | 0 |
| Cerasorb M | 99.9675 | 12.555 | 2.15 | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 | 0.49 |
| Standard Deviation | 34.8 | 3.2 | 0.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The vancomycin release-level followed a similar pattern, although the vancomycin concentration eluted by Cerasorb M® granules stayed above the detection threshold during the period of the experimental (Fig. 2, Table 2).
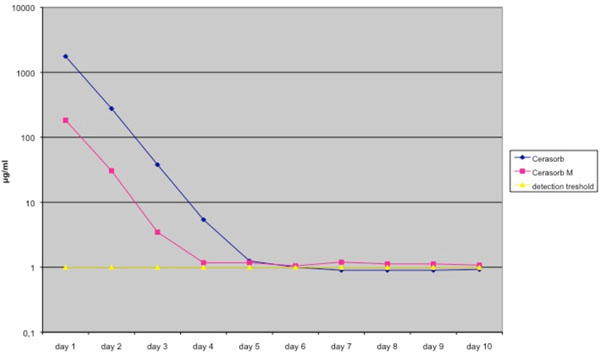
Elution pharmacokinetics of vancomycin from Cerasorb® and Cerasorb M®. Antibiotic release is expressed in μg /ml. Mean values were derived from a total of 4 different tests. Granules were loaded in a solution of 50 g/L antibiotic.
Elution Pharmacokinetics and Standard Deviation of Vancomycin from Cerasorb® and Cerasorb M®. Antibiotic Release is Expressed in µg /ml. Mean Values were Derived from a Total of 4 Different Tests. Granules were Loaded in a Solution of 50 g/L Antibiotics
| Elution Kinetics of Vancomycin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | Day 8 | Day 9 | Day 10 | |
| Cerasorb | 1761.3 | 277.35 | 37.875 | 5.4 | 1.25 | 1 | 0.9 | 0.9 | 0.9 | 0.925 |
| standard deviation | 331.87 | 32.46 | 2.55 | 0.41 | 0.25 | 0.2 | 0 | 0 | 0 | 0.05 |
| Cerasorb M | 182.775 | 30.55 | 3.475 | 1.175 | 1.175 | 1.05 | 1.2 | 1.125 | 1.125 | 1.075 |
| standard deviation | 27.9 | 23.4 | 0.56 | 0.4 | 0.3 | 0.3 | 0.38 | 0.45 | 0.45 | 0.35 |
| detection treshold | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
DISCUSSION
Osteomyelitis is a difficult infection to treat and to eradicate. It represents a challenge for orthopaedic surgeons worldwide. Multiple surgical debridements and long-term parental antibiotics are often required for effective therapy. Besides these techniques, the local administration of antibiotics plays a crucial role in a successful therapy.
The main goal for local antibiotic therapies is to deliver an effective antimicrobial at sufficiently high concentrations to the area of infection. It is usually performed in addition with the use of polymethylmetacrylate (PMMA) beads and bone cements. The disadvantages associated with the usage of PMMA beads include being determined to one antibiotic at a time and the necessity of a second surgery to remove the beads after the antibiotic elution [2, 3, 21].
All these disadvantages made an intense search for possible alternatives necessary. An ideal antibiotic delivery system should provide an adequate antimicrobial concentration at the target site a constant release of antimicrobial over a prolonged period and be biodegradable to avoid the necessity of a second surgery [30].
One possible alternative is calciumsulphate, which is, as several studies showed, an adequate local drug delivery system [11, 12]. Admittedly during the first 60 days after implantation calciumsulphate causes inflammatory reactions in the circumjacent tissue [8]. After day 60 the inflammation ceases in the affected bone, but not in the circumjacent soft tissue.
Lactic-acid polymer gained interest as antibiotic carrier material. These polymers are biodegradable, making a second surgery to remove the carrier material unnecessary. In vitro, as well as in vivo, lactic-acid polymers showed a high release kinetic of loaded antibiotics [22-25]. A big drawback is its nonexistent osteoconductive property. Lactic-acid polymers are not commercially available. The addition of nanocrystalline hydroxyapatite to calciumsulphate enhanced the biocompatibility of calciumsulphate in a composite material. This combination showed a reduced inflammatory reaction in the adjacent soft tissue in comparison to pure calciumsulphate, as well as higher osteoconductive properties and provides a scaffold for new bone formation [26]. In vitro the calciumsulphate hydroxyapatite composite showed a high release rate of the loaded antibiotics, in particular during the first days of the trial this antibiotic delivery system showed local effective antibiotic concentrations above the minimal inhibiting concentration (MIC) of vancomycin and gentamicin susceptible pathogens [14]. The most relevant pathogen for bone infections is Staph. Aureus, showing an MIC90 (antibiotic concentration that inhibits growth of 90% Staph. Aureus strains) of 1 mg/L for both vancomycin and gentamicin in susceptible strains [14, 23, 27].
Calcium sulphate preloaded with Tobramycin (OsteoSet®T) in combination with surgical debridement showed promising results in the treatment of infected bone defects. However, an individualized chemotherapy in terms of local administration of different antibiotics is not provided [12]. To avoid the emerging tobramycin resistance custom-made calciumsulphate pellets, individually loaded with the appropriate antibiotic, are commercially available. In clinical and experimental studies these pellets showed a high efficiency in treating osteomyelitis. However, antibiotic loading has to be done before hardening and sterilization, creating a potential risk of reducing antibiotic efficiency. Adding the antibiotic during the process of hardening may impair the antibiotic elution. Dacquet et al. showed that the elution of teicoplanin, which was added to the calcium sulphate powder before hardening, was only 1-5 μg during the first 10 days, too low for an effective osteomyelitis treatment [11]. Furthermore, there is a potential deceleration in the surgical procedure due to the 12-14 minutes of hardening.
In the current study we tried to minimize possible risks of impairing antibiotic activity due to any hardening or sterilization processes. To prevent inactivation of antibiotics by hardening or sterilization we performed the loading of antibiotics after these procedures. The ready-for use pellets completely absorbed the antibiotic solution. By comparing Cerasorb® and Cerasorb® M with their different porosities, we proved that antibiotic uptake and release are influenced by different porosities of the bone graft pellets. The higher porosity of Cerasorb® M showed a lower release of both loaded antibiotics from the start. The antibiotic concentrations never reached the antibiotic concentrations eluted by Cerasorb®. This result is in accordance with several other studies [14, 20]. Based on a minimal inhibition concentration (MIC) of 1 mg/L for both vancomycin and gentamicin, both materials achieved effective local antibiotic concentrations for Staph aureus within the first 4 and 5 days, respectively. After day 4 and 5, respectively, for both tested bone graft materials local antibiotic concentrations were less than the MIC and under the detection treshold. Rauschmann et al. reported similar results for the elution of vancomyin and gentamicin with nanocrystaline hydroxyapatite calcium sulphate as a carrier material. Local antibiotic concentrations for both antibiotics were initially high and stayed above the detection treshold for 3-4 days [14]. The present study showed concentrations of released gentamicin and vancomycin exceeding 100-1000-fold the MIC of susceptible Staph. aureus within the first day, at day 2 and 3 the MIC was exceeded 10-100-fold.
Prima facie these results seem to be comparable with the results reported by Rauschmann et al., however, the main difference is the method to measure the antibiotic concentration. Rauschmann et al. used a standard agar diffusion test with Bacillus subtilis ATCC 6633. In contrast, we used machine-aided diagnosis to evaluate the antibiotic elution of both used bone graft substitutes [14]. Wichelhaus et al. used the standard agar diffusion test as well in their evaluation of the release of 4 different antibiotics from calcium sulphate carrier beads and found antibiotic release to be above the MIC for a period of ten days [20].
Miclau et al. showed a high initial antibiotic release from autogenic bone graft and demineralized bone matrix during the first days of their study, whereas PMMA- and calcium sulphate carrier beads showed a prolonged release of the loaded antibiotics [29]. A major drawback of autogenic bone graft is the required removal of the bone graft resulting in a high risk for complications, as well as its limited disposability. Demineralized bone matrix is not rugged and due to its structure is limited in the way it acts as an osteoconductive scaffold.
In contrast calcium phosphate ceramics, like hydroxyapatite and tricalciumphoshate, are available in large quantities and show excellent osteoconductive features. Different studies have proven the qualification of hydroxyapatite as a local antibiotic delivery system [28, 30].
CONCLUSION
The tricalcium phosphate ceramics Cerasorb® and Cerasorb® M are well established as bone graft substitutes. In the current study we loaded both ceramics with vancomycin and gentamycin to create a local antibiotic delivery system. The pre-fabricated pellets were loaded after sterilization and hardening to prevent inactivation of antibiotics by these procedures. We showed a high initial antibiotic release with subsequent decline well above the MIC of Staph aureus for both materials within the first 4 and 5 days, respectively. The pre-fabricated pellets offer fast and technically easy loading with the selected antibiotic. Loading can be done according to antibiograms offering individualized antibiotic treatment options. Due to the osteoconductive and biodegradability properties of Cerasorb® and Cerasorb M® there is nor need for removal of the material as in the case with PMMA. Given the different technical features of both ceramics it enables practitioners to react to different clinical requirements, e.g. slower or faster resorption.
In conclusion Cerasorb® and Cersorb M® has potential as a new treatment option in osteomyelitis and infectious bone disease.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported in part by Curasan AG, Kleinostheim, Germany.


