All published articles of this journal are available on ScienceDirect.
Quantification of Vertebral Involvement in Metastatic Spinal Disease
Abstract
Introduction:
For patients with a solitary and well-delimitated spinal metastasis that resides inside the vertebral body, without vertebral canal invasion, and who are in good general health with a long life expectancy, en bloc spondylectomy/total vertebrectomy combined with the use of primary stabilizing instrumentation has been advocated. However, clinical experience suggests that these qualifying conditions occur very rarely.
Objective:
The purpose of this paper is to quantify the distribution of vertebral involvement in spinal metastases and determine the frequency with which patients can be considered candidates for radical surgery (en bloc spondylectomy).
Methods:
Consecutive patients were classified accordingly to Enneking’s and Tomita’s schemes for grading vertebral involvement of metastases.
Results:
Fifty-one (51) consecutive patients were evaluated. Eighty-three percent of patients presented with the involvement of multiple vertebral levels and/or spinal canal invasion.
Conclusion:
Because of diffuse vertebral involvement of metastases, no patients in this sample were considered to be candidates for radical spondylectomy of vertebral metastasis.
INTRODUCTION
In an aging population, chronic degenerative diseases and cancer have been highlighted as major causes of morbidity and mortality [1-8].
Up to 40% of cancer patients will develop skeletal metastases; the spine, due to its size, contiguity and rich vascularization, is the primary affected bone site [1-3]. Among patients who develop spinal metastases, only 5%-10% will develop epidural spinal cord compression, and 10% of those patients will be symptomatic [1-6]. The number of bone metastases increases with prolonged patient survival, and these metastases are derived from primary tumors originating from the kidney, breast, prostate and other organs [1-9].
The proposed surgical treatment of spinal metastases is controversial. Although non-operative treatments and adjuvant therapies remain important options, surgical strategies that include the entire range of operative procedures should also be considered [9-15].
En bloc spondylectomy/total vertebrectomy accompanied by reconstruction with primary stabilizing instrumentation has been advocated for patients who meet the following criteria: the presence of a solitary and well-delimitated spinal metastasis that resides inside the vertebral body without vertebral canal invasion, good general health, and a long life expectancy [9-12]. However, clinical experience suggests that these qualifying conditions occur very rarely.
The purpose of this paper is to quantify the distribution of vertebral involvement in spinal metastases and to determine the frequency with which patients requiring admission due to spinal metastasis can be considered as candidates for radical surgery (en bloc spondylectomy).
MATERIALS AND METHODOLOGY
Vertebral Metastasis Involvement Classification Scheme
An electronic literature search was performed to reveal the published classifications used to quantify vertebral metastasis involvement. The following search strategy was used: ("spine"[MeSH Terms] OR "spine"[All Fields] OR "vertebral"[All Fields]) AND ("neoplasm metastasis"[MeSH Terms] OR ("neoplasm"[All Fields] AND "metastasis"[All Fields]) OR "neoplasm metastasis"[All Fields] OR "metastasis"[All Fields]) AND ("classification"[Subheading] OR "classification"[All Fields] OR "classification"[MeSH Terms]). Classification schemes that we considered appropriate for grading the involvement of metastatic spinal disease were analyzed, and cross-references for vertebral classification schemes were searched. From these, Enneking, Tomita, Weinstein-Boriani-Biagini (WBB) and Harrington scales for vertebral involvement were initially considered for our quantification of vertebral involvement [4, 14-19].
The WBB classification was excluded from consideration because we deemed it to be extremely compartmentalized and adequate only for slow-growing tumors, such as benign and primary bone tumors. Harrington’s classification was designed to evaluate vertebral stability, and although it describes vertebral collapse and stability, it embodies other data that are not specific toward determination of vertebral involvement; thus, this classification system was also excluded from consideration.
Ultimately, patients were classified accordingly to Enneking’s (Fig. 1) and Tomita’s (Fig. 2) schemes for vertebral involvement classification.

Diagram based on Enneking’s classification of benign tumors. The top three diagrams represent benign tumors and the lower four, malignant tumors. 1, tumor capsule; 2, tissue reaction; 3, tumor island within adjacent tissue reaction; 4, skip metastasis.
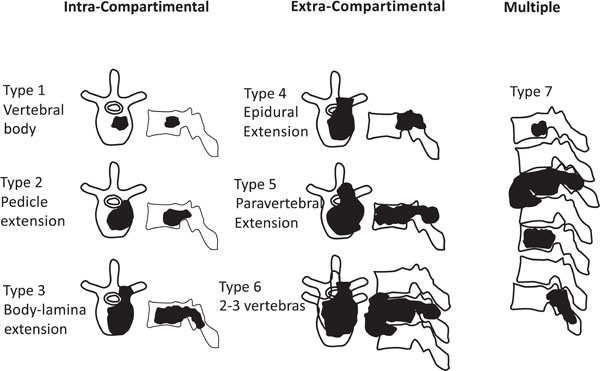
Diagram based on Tomita´s classification of vertebral metastasis.
PATIENTS AND METHODS
The study sample consisted of patients with vertebral metastases requiring admission consecutively admitted from July 2010 to October 2012 at the Hospital do Servidor Público Estadual de São Paulo (HSPE). We set the number of patients to be studied as the number that represent a normal sample patient distribution and defined the sample between 30 and 50 patients due to possible losses during study.
This project was approved by the Research and Ethics Committee of HSPE.
The patients received complete clinical and neurological examinations and were classified according to the Karnofsky scale and the Frankel scale, respectively.
STATISTICS
Numerical data were described as the means ± standard deviations. Categorical data are presented as percentages. To determine the distribution of our data, the Kolmogorov-Smirnov Test was used. Student's t-test was used for the paired and unpaired groups as appropriate. The significance level was established as p <0.05.
RESULTS
Fifty-one consecutive patients with spinal metastases who were admitted to the Hospital do Servidor Público Estadual de São Paulo (HSPE) were evaluated between July 2010 and October 2012. Sixteen patients were female, and 35 were male. The average age was 61.07 ± 11.78 for women and 62.74 ± 10.17 for men. The ages of the groups did not differ significantly (p> 0.05).
Of the 51 patients, only 1 was asymptomatic and was referred from the oncology department after an active search for metastases. Fifteen patients presented with spinal pain, 17 with neurological deficits and 18 with both pain and neurological deficits.
All patients were neurologically (Frankel scale; Fig. 3) and clinically (Karnofsky scale; Fig. 4) evaluated. Neurologically, 5 patients presented with a complete deficit (Frankel A), 2 with Frankel B, 19 with Frankel C, 9 with Frankel D and 16 with Frankel E (Fig. 3).
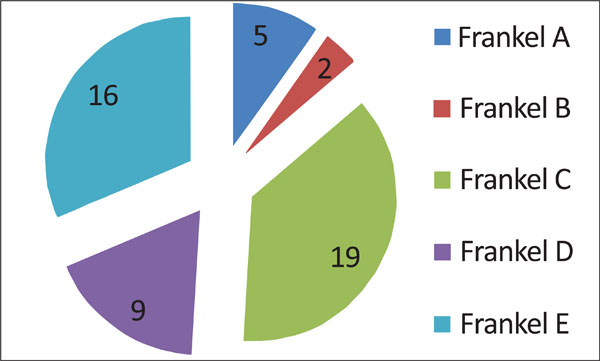
Neurological patient presentation based on Frankel scale. Grade A: Complete neurological injury with no motor or sensory function clinically detected below the level of the injury. Grade B: Preserved sensation only; no motor function clinically detected below the level of the injury; sensory function remaining below the level of the injury but may include only partial function. Grade C: Preserved motor non-functional. Grade D: Useful motor function below the level of the injury; patient can move lower limbs and walk with or without aid but does not have a normal gait or strength in all motor groups. Grade E: Normal motor functioning.
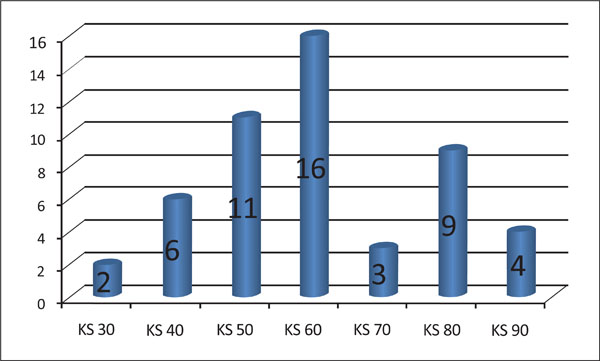
Number of patients corresponding to each grade of the Karnovsky scale (KS).
The KS varied from 30 to 90. Two patients presented with a KS of 30, six patients with a score of 40, eleven patients with a score of 50, sixteen patients with a score of 60, three patients with a score of 70, nine patients with a score of 80 and four patients with a score of 90 (Fig. 4).
Vertebral metastases were localized in the thoracic spine in 82% of cases, the lumbar spine in 50%, the cervical spine in 26% and the sacral spine in 10%.
All patients had a known histopathological diagnosis (Fig. 5). Twelve were diagnosed with primary tumors in the breast, twelve in the prostate, and four in the lung. Four patients had multiple myeloma, three had colon cancer, and three had non-Hodgkin’s lymphoma. Bladder, kidney and larynx cancers were reported by one patient each.
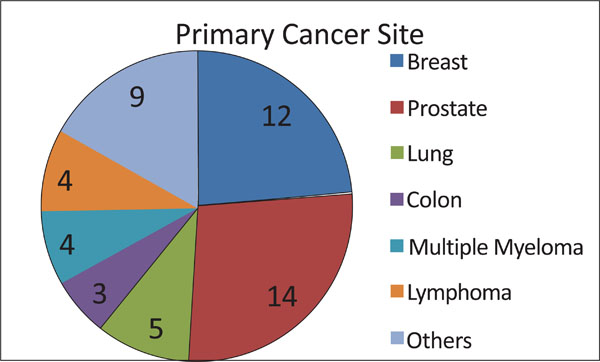
Number of patients with vertebral metastasis from each primary tumor type.
VERTEBRAL SPINE INVOLVEMENT
Enneking’s Classification
All of the patients had vertebral involvement above Enneking’s IIA level (Fig. 6). Each patient had a tumor extending abroad from the cortical vertebral body limits. Due to the extension of vertebral body involvement, it was not possible to identify skip metastases inside the vertebral body as has been performed for benign spine tumors. The highest grade in this classification scheme (Enneking’s 3) should be given to all patients in this study (Fig. 1).
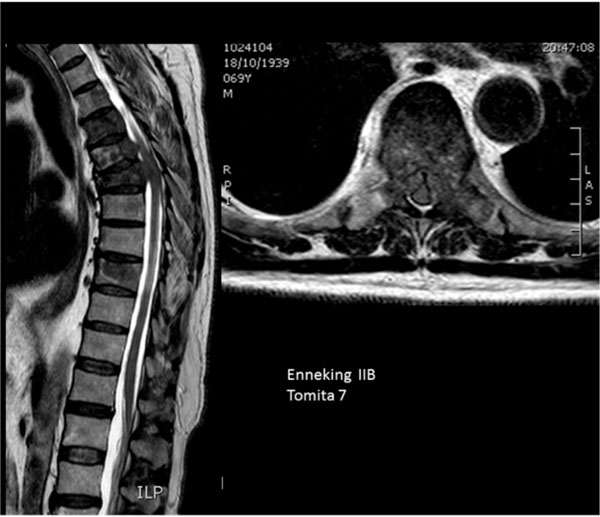
Multiple vertebral involvement (Tomita’s grade 7).
Tomita’s Classification
Eighty percent of patients (35) were classified with Tomita’s grade 7 (involvement of multiple vertebrae (Fig. 7)).
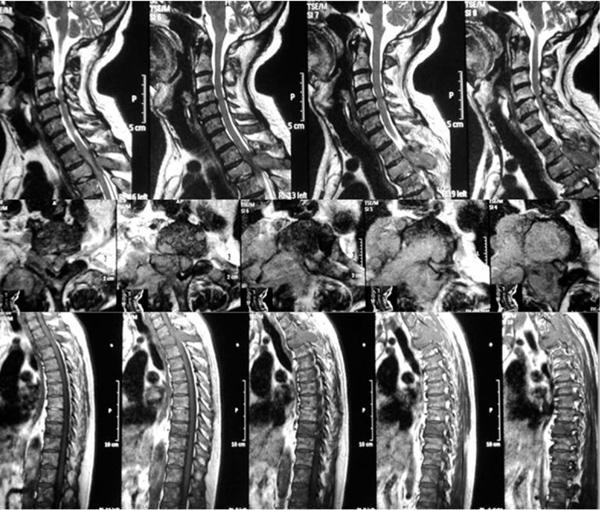
All spine is involved in vertebral metastasis.
Seventeen percent of patients (8 out of 516) were assigned to Tomita’s Grade 6 (involvement of two or three vertebrae), and only one patient (2%) was designated with Tomita’s grade 5 (single level with paravertebral and spinal canal involvement) (Fig. 8). Only one patient presented with a Tomita grade 1 vertebral level of tumor invasion. Two patients presented with two levels, five patients with three levels, five patients with four levels, one patient with five levels and 30 patients with more than 5 levels.
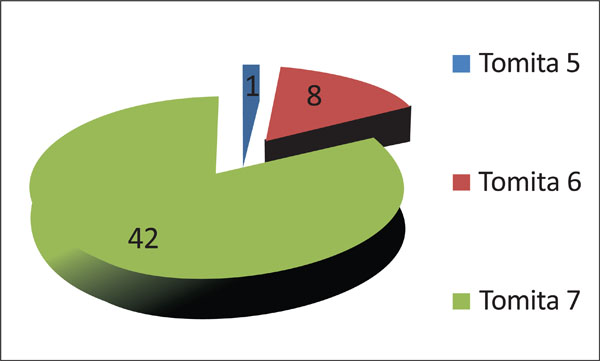
Classification of patients according to Tomita’s vertebral involvement scale.
The spinal canal was invaded in 83% of the patients, being spared in only 8 patients, in whom metastatic involvement was concentrated in the bone structures.
Forty-five percent (45%) of patients were treated conservatively with radiotherapy, and forty-three (43%) were treated with a decompression-only approach. In 6% of patients, decompression was combined with spine fixation, and 6% of patients received only a diagnostic percutaneous biopsy. No patients were treated with radical excision surgery.
DISCUSSION
Vertebral involvement quantification, vertebral canal invasion, neurological status, general health status and the malignancy prognosis, determined by primary tumor histology, are paramount factors to consider for surgical planning and establishing therapeutic targets. A variety of surgical methods are available to treat spinal metastases. Dorsal spinal decompression and stabilization are the most frequent surgical techniques used to treat metastatic disease of the thoracic and lumbar spine [1, 2, 9, 12, 20-24]. Because >60% of spinal metastases are hypervascular, preoperative embolization may also be considered in order to decrease hemorrhage risk and improve outcomes with low complication rates [25, 26].
For patients with a solitary spinal metastasis without vertebral canal invasion and who are in good general health with a long life expectancy, ventral tumor resection (en bloc spondylectomy/total vertebrectomy) accompanied with primary stabilizing instrumentation has been suggested [9, 10, 24, 27].
According to Tomita’s study, for patients with a prognostic score of 2 or 3, the treatment goal is long-term local control, with an expected survival period of more than 2 years. For these patients, wide marginal excision (en bloc spondylectomy) is appropriate [16].
When the treatment goal is middle-term local control (prognostic score of 4 or 5 on Tomita’s scale), intralesional excision methods such as piecemeal excision or eggshell curettage are the appropriate surgical modalities. For patients with a prognostic score of 6 or 7, palliative surgery such as spinal cord decompression with stabilization is the first choice for short-term palliation, and when the prognostic score is 8, 9, or 10, supportive care is advocated [16].
Although several prognostic vertebral metastasis classifications [27-29] have been published, we were able to identify only two classification schemes for quantification of vertebral body involvement [4, 15-19, 30]. Because all patients were consistent with the maximal possible grade in Enneking’s classification (considering that a skip metastasis is not an adequate criteria for spine metastasis), this classification does not discriminate between several possible spinal involvements and was not adequate for studying vertebral metastasis. Tomita’s classification is the adequate classification to grade spine vertebral metastasis involvement.
Cancer patients with metastases are challenging to treat because metastasis represents an advanced stage of disease and, hence, a poor prognosis [1-3, 9, 20, 21]. The majority of the patients in our study presented with poor-to-moderate general health conditions as stratified by KS. In addition, many of the patients (83%) presented with involvement of multiple vertebral levels and extensions to the spinal canal.
Almost seventy percent (69,5%) of patients presented with some neurologic deficit.
None of the fifty-one patients in our study were found to be candidates for oncologic surgery (radical tumor resection).
However, we have only included patients requiring Hospital admission and a large portion of them were quite ill and/or neurologically impaired. It is possible that an unknown percentage of outpatients may be in better clinical condition and thus be candidates to a more radical surgery.
Therefore, candidates for radical en bloc surgery must exist at a frequency of less than 1/51. Furthermore, due to the nature of vertebral metastasis dissemination, these hypothetical patients can be considered candidates for the surgery for only a short duration of their cancer disease if the primary emboligenic cancer focus is not completely eradicated.
CONCLUSIONS
Due to diffuse vertebral involvement of metastases no patients in this sample could be considered candidates for radical spondylectomy of vertebral metastasis.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


