RESEARCH ARTICLE
Intrasynovial Tendon Graft for Chronic Flexor Tendon Laceration of the Finger: A Case Report
Jun Sasaki, Toshiro Itsubo, Koichi Nakamura, Masanori Hayashi, Shigeharu Uchiyama*, Hiroyuki Kato
Article Information
Identifiers and Pagination:
Year: 2013Volume: 7
First Page: 282
Last Page: 285
Publisher ID: TOORTHJ-7-282
DOI: 10.2174/1874325001307010282
Article History:
Received Date: 24/1/2013Revision Received Date: 8/6/2013
Acceptance Date: 9/6/2013
Electronic publication date: 19/8/2013
Collection year: 2013

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/) which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
We present the case of a patient with flexor digitorum profundus tendon laceration at the A2 pulley level caused by an injury to the base of the right ring finger by a knife. The patient was treated by flexor tendon reconstruction from the palm to the fingertip by using the left second toe flexor tendon as a graft, which improved the active range of motion. Further improvement was achieved by subsequent tenolysis, which eventually restored nearly normal function. Our experience with this case indicates that the intrasynovial tendon is a reasonable graft source for the synovial space in fingers and may enable restoration of excellent postoperative function.
INTRODUCTION
Flexor tendon grafting in patients with chronic flexor tendon injury in the finger does not always provide satisfactory outcomes [1-4]. The outcomes are influenced by the surgical technique [2,5], state of the digital pulleys or the neurovascular bundles [1], passive range of motion (ROM) of the finger joints [6], age of the patient [3,4], and adherence to the postoperative rehabilitation program [7,8]. On the other hand, it remains to be determined whether the source of the graft affects the postoperative outcomes. The palmaris longus tendon and the plantaris tendon, both of which are extrasynovial tendons, are the most popular graft sources used for flexor tendon reconstruction because they are easy to harvest without donor site morbidity. However, intrasynovial tendon grafts have been theoretically regarded as a more ideal source of tendon grafts since these grafts have been shown to have superior biologic and biomechanic properties compared to extrasynovial tendon grafts in the synovial space [9-12]. Leversedge et al. first reported the outcomes in 8 cases of flexor tendon injuries, wherein the second toe flexor tendon was utilized as the intrasynovial graft [13]; according to their study, the outcomes have been favorable, but not necessarily superior to those of the conventional procedure. No subsequent reports have been published on intrasynovial tendon grafts. Here, we report the excellent outcomes associated with intrasynovial tendon grafting in a patient with chronic flexor tendon laceration of the finger. The patient was informed that the case would be submitted for publication, and his consent was obtained.
CASE REPORT
The patient was a 25-year-old, right-handed man presenting with limited flexion of his right ring finger. He had sustained a knife injury at the base of the right ring finger. He visited a surgeon at a local clinic, who sutured the wound; the patient was then informed that the flexor tendon was intact. However, since the limited flexion persisted even 4 months after the injury, he was referred to our hospital. On examination, the active ROM of the right ring finger was found to be 88°, 68°, and 12° of flexion and 0°, 10°, and 0° of extension for the metacarpophalangeal (MP) joint, proximal interphalangeal (PIP) joint, and distal interphalangeal (DIP) joint, respectively. The total active motion (TAM) was 168° (percent TAM, 62%) (Fig. 1). Grip strength was 24 kg for the right hand and 41 kg for the left hand. The disabilities of the arm, shoulder, and hand (DASH) score was 31 points.
The patient was diagnosed with flexor digitorum profundus (FDP) tendon laceration at the A2 pulley level. Flexor tendon reconstruction from the palm to the fingertip was then performed using the left second-toe flexor tendon as a graft at 5.5 months after the injury, under general anesthesia. The left side was chosen because his right foot was dominant. Through a volar zigzag skin incision, the involved flexor system was exposed from the A2 to the A5 pulleys. All pulleys were intact. Frayed and degenerated distal stump and enlargement of the swollen proximal stump of the FDP tendon were noted (Fig. 2). The flexor digitorum superficialis (FDS) tendon was found to have been transected and attached to the PIP joint volar plate. A separate skin incision was made over the A1 pulley to locate the proximal stump of the FDP tendon.
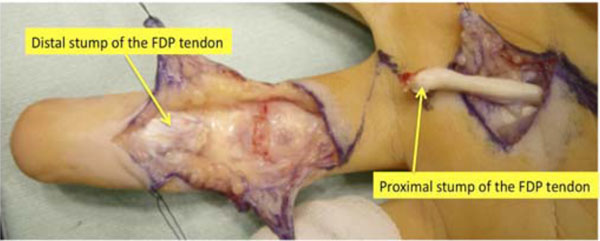 |
Fig. (2). The distal stump of the flexor digitorum profundus tendon is frayed and degenerated. The proximal stump is swollen and degenerated. FDP: flexor digitorum profundus, |
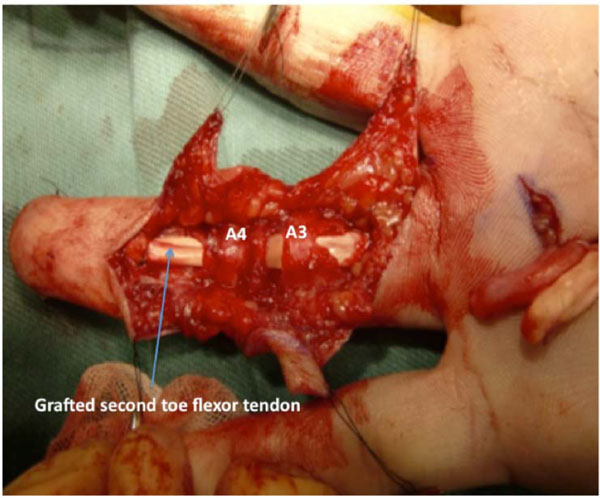
The left second-toe flexor tendon was harvested by the method described by Leversedge et al. [13]. Thereafter, the grafted tendon was advanced distally through a series of fibrous pulleys in the ring finger. Since the graft was not easily maneuvered through the A2 pulley with the FDS tendon attached to the volar plate, the FDS tendon was cut. The size and shape of the graft tendon were suitable for the ring finger synovial space. A distal juncture was created by a modification of the classic Bunnell tendon-to-bone pull-out technique. For the proximal juncture, 3 weaves of the grafted tendon were applied to the proximal stump of the FDP tendon (Fig. 3).
On postoperative day 1, modified Kleinert’s early motion was initiated with a dorsal extension block splint, and active flexion was initiated 3 weeks after surgery. At 7 months after surgery, the TAM was 194° (percent TAM: 71%). Magnetic resonance imaging (MRI) of the ring finger revealed that the configuration of the grafted tendon was almost normal (Fig. 4). Since the patient wished to gain further active flexion of the right ring finger, tenolysis was performed 7 months after surgery. The appearance of the graft tendon from the second toe was normal, without apparent degenerative findings (Fig. 5). Adhesion was observed from the palm to the insertion point at the base of the distal phalanx, especially, at the proximal juncture site in the extrasynovial area. Release of the grafted tendon from the surrounding structures was easily accomplished. Immediate active ROM exercises were encouraged. At 1.5 years after tenolysis, the active ROM was 90° of flexion and 12° of extension for the MP joint, 100° of flexion and 16° of extension for the PIP joint, and 80° of flexion and -2° of extension for the DIP joint. TAM was 268° (percent TAM: 99%) (Fig. 6). Grip strength was 46 kg for the right hand. The DASH score was 5.8 points. Although limited flexion of the second toe was evident, the patient’s gait was normal and he experienced no further morbidity at the donor site.
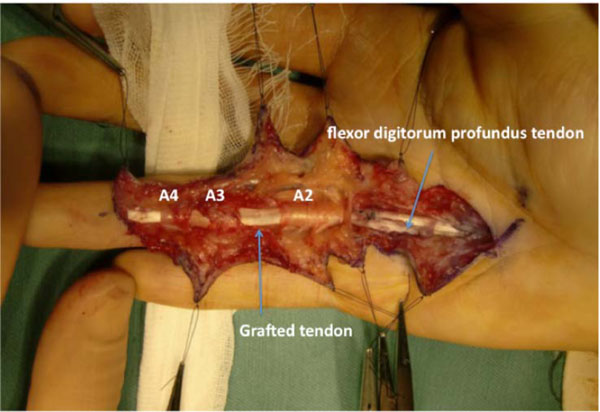 |
Fig. (5). The grafted tendon after tenolysis, with an appearance similar to a normal FDP tendon. |
 |
Fig. (6). Active motion of the ring finger at 1.5 years after tenolysis. Note the nearly full extension (A) and flexion (B). TAM was 268°. |
DISCUSSION
Previous experimental studies have shown that intrasynovial tendon grafts have 2 distinct advantages over extrasynovial tendon grafts, both in vivo and in vitro. An in vivo animal study in dogs showed that an intrasynovial tendon graft within the digital sheath was histologically viable and normal, and that no adhesion occurred between the grafted tendon and the digital sheath at the so-called no man’s land [14]. This early finding was further verified in the 1990s. Use of canine models clearly demonstrated that compared to extrasynovial tendon grafts, intrasynovial tendon grafts are associated with less tenocyte death and less adhesion around the tendon. These observations are consistent with those noted an in vitro study on a human cadaver, which showed that the FDP tendon glides through the A2 pulley with less friction than the palmaris longus tendon [15].
We assumed that the limited improvement in digital motion after the first surgery was due to the adhesion of the grafted tendon at the proximal suture site in the palm. Since the subsequent MRI showed that the configuration of the grafted tendon was almost normal, with normal low signal intensity, we expected that tenolysis would result in normal gliding with good transmission of force. Adhesions were noted throughout the synovial space, up to the proximal suture site in the palm of the extrasynovial area, but those in the latter area were of the greatest significance. This observation is consistent with the findings of the abovementioned animal studies and those in the cases of tenolysis after intrasynovial tendon grafting reported by Leversedge et al. [13].
Hyperextension of the MP and PIP joints was possible for our patient, indicating nearly normal function of the ring finger after the second surgery. The active ROM in our patient may be regarded as one of the best results of flexor tendon reconstruction in adults, when considering the findings of recent case series [16-18]. We have never previously experienced such a significant improvement with conventional extrasynovial tendon grafting, even in children [4]. However, the results of conventional tendon grafting in our case may have been comparable to that of intrasynovial tendon grafting because of the good condition of the patient.
Patient-oriented questionnaires have rarely been used for the assessment of outcomes after flexor tendon reconstruction [18]. Such questionnaires should also facilitate the evaluation of the outcomes of tendon surgery. We observed a significant improvement in the DASH score in our patient, which we believe is attributable to the successful functional recovery of the ring finger.
The sheath mechanism of the second-toe flexor tendon is known to be similar to that of the finger tendons, and the length of the second-toe flexor tendon has been shown to be sufficient for fingers, if the proximal suture is placed in the palm [19]. Seiler et al. also showed that the intrasynovial tendons of the hand and foot are similar in vascularity, cellularity, gliding surface, and ultrastructure [20].
Compared to second-toe intrasynovial tendon grafting, conventional extrasynovial tendon grafting is less invasive in terms of donor-site morbidity. In fact, our patient did experience some donor-site morbidity, namely, loss of flexion of the second toe interphalangeal joints. However, the patient did not complain of any pain in the foot or the toe and can walk and even run without any difficulties. Despite the limited donor-site morbidity associated with the technique, sparing the second-toe flexor tendon would have been preferable for digital flexor tendon reconstruction. An alternative procedure for tendon grafting involves modifying the surface of the extrasynovial tendon; a tendon substitute has also been extensively investigated [21, 22]. However, until these procedures are available for clinical use, the intrasynovial tendon can serve as a reasonable graft source for the synovial space in fingers and may enable restoration of excellent postoperative function.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.