All published articles of this journal are available on ScienceDirect.
Tartrate-Resistant Acid Phosphatase 5b is a Useful Serum Marker for Diagnosis and Recurrence Detection of Giant Cell Tumor of Bone
Abstract
Serum tartrate-resistant acid phosphatase (TRACP) 5b was investigated for use as a marker for diagnosis of giant cell tumor (GCT) of bone and for detection of its recurrence.
Four patients with GCT of bone who were initially referred to our hospital were classified as a primary group. Three patients who had local recurrence following curettage were classified as a local recurrence group. Five with no recurrence were classified as a no-recurrence group. Eighteen patients with primary and metastatic malignant bone tumors were also enrolled in the study as a control group. Serum TRACP 5b was measured before the biopsy in all patients and was measured periodically after the operation in patients with GCT of bone. Student t-tests were used for statistical analyses.
TRACP 5b was greater than 1500 Um/dL in all primary group patients. Mean TRACP 5b values decreased gradually with post-operative time, showing lower values until local recurrence. The mean value of TRACP 5b of the local recurrence group (753 ± 68.7 mU/dL) was significantly higher than that of the no-recurrence group (340.6 ± 78.3 mU/dL). The mean value of TRACP 5b of the control group (466.9 ± 130.3 mU/dL) was much lower than that of the primary group and markedly lower than that of the local recurrence group. However, no significant difference was found between the no-recurrence group and the control group.
Serum TRACP 5b is a useful and convenient marker for diagnosing GCT of bone and for predicting its recurrence.
INTRODUCTION
Giant-cell tumor (GCT) of bone is an extremely common primary bone tumor that occurs mostly at the epiphyseal region of long bones in patients at the age of 20-40 years [1]. Although radiological modalities such as plain radiography, computed tomography (CT), and magnetic resonance imaging (MRI) are used for its diagnosis, histological analysis must be conducted for a definite diagnosis [1, 2]. Curettage is the most popular treatment of the tumor [1-5]. However, local recurrence following curettage is reported [1, 6]. Furthermore, earlier detection of local recurrence by careful follow-up is important before an opportunity for successful re-curettage is lost [6]. Multinucleated cells of giant cell tumors contain acid phosphatase of a specific secretory type [7]. Serum acid phosphatase values have been used for diagnosis and for evaluation of the efficacy of treatment for GCT of bone [8-10].
Acid phosphatases are discriminated by tartrate, which is the most effective inhibitor of prostatic acid phosphatase. Cells of monocyte origin, including macrophages, dendritic cells, and osteoclasts, express abundant tartrate-resistant acid phosphatase (TRACP) in mammals [11]. Apparently, osteoclasts mostly express type 5b of TRACP in the serum. Osteoclasts secrete TRACP 5b, implying that serum TRACP 5b has unique specificity as a marker of osteoclast activity, and that it is therefore a useful marker of bone resorption [11, 12]. Based on those facts, serum TRACP 5b has been used clinically as a serum marker for diagnosing osteoporosis. Recently, serum TRACP 5b has been used clinically as a specific and sensitive marker of bone resorption for the diagnosis of cancer patients with bone metastasis [11, 13, 14], for the evaluation of the aggressiveness of osteosarcoma [15], and as a marker of late loosening of total hip arthroplasty [16]. Histologically, GCT of bone consists of uniform mononuclear cells, with osteoclast-like giant cells scattered among them [1, 17]. These facts suggest that serum TRACP 5b values have some relation to GCT of bone. This study was undertaken to investigate the usefulness of serum TRACP 5b as a specific marker for both the diagnosis and recurrence of GCT of bone.
MATERIALS AND METHODS
The Institutional Ethics Committee on human research of the university approved the study design. All patients who were referred to our clinic presenting the osteolytic lesion and who had been diagnosed with histologically confirmed GCT of bone during March 2009 - April 2011 were enrolled in this study. Blood chemistry data, including those for TRACP 5b, were obtained before operation. After the operative treatment, serum TRACP 5b was followed by plain radiography, computed tomography (CT), and MR imaging to assess the respective probabilities of local recurrence and pulmonary metastases. For cases in which local recurrence was suspected from radiological findings, serum TRACP 5b was measured following operative treatment.
As a primary group, four patients were referred initially to our hospital for bone tumor treatment. They were subsequently diagnosed as having GCT of bone based on histology of the needle biopsy. The one man and three women had mean age of 43 years (21-74) at the time of enrollment (Table 1). Of these four GCT of bone cases, two were located in the distal femur, one in the proximal tibia, and one in the distal radius. No pulmonary metastasis was observed in any patient. Three patients underwent curettage without bone graft. One underwent wide resection.
Clinical Characteristics of GCT of Bone Patients
| Case No.# | Age | Gender | Location | Situation* | Operative Procedure | Follow up Period** | TRACP 5b |
|---|---|---|---|---|---|---|---|
| 1 | 21 | F | proximal tibia | primary | curettage | / | 1500 <*** |
| 2 | 26 | M | distal femur | primary | curettage | / | 1500 < |
| 3 | 51 | F | distal femur | primary | curettage## | / | 1500 < |
| 4 | 74 | F | distal radius | primary | wide resection | / | 1500 < |
| 5 | 52 | F | distal radius | local reccurence | curettage | 8Y | 656 |
| 6 | 36 | F | proxima tibia | local reccurence | curettage | 16Y 5M | 856 |
| 7 | 43 | F | proximal tibia | local reccurence | curettage | 6Y 2M | 747 |
| 8 | 38 | M | distal femur | no reccurence | curettage | 3Y 4M | 332 |
| 9 | 25 | F | distal femur | no reccurence | curettage | 4Y 8M | 493 |
| 10 | 34 | M | proximal humerus | no reccurence | curettage | 4Y 1M | 326 |
| 11 | 40 | F | ischium | no reccurence | curettage | 4Y 3M | 168 |
| 12 | 67 | M | proximal humerus | no reccurence | wide resection | 9Y 5M | 384 |
# No pulmonary metastasis was observed in any patient.
* Patients without any prior treatment are 'primary', those with operative treatment following local reccurence are 'local reccurence', and those with operative treatment without local reccurence are 'no reccurence'.
** Duration between the operative treatment and the occurrence of the local recurrence (local recurrence group) or the measurement of TRACP 5b (no recurrence group). Y, year; M, month.
*** Measurement data are more than 1500 mU/dL.
## Pathological fracture occurred during curettage.
Patients with GCT of bone who underwent curettage without bone graft at our hospital before March 2009 and who were referred to our clinic for follow-up during that period were also enrolled in the study as a local recurrence group (Table 1). No pulmonary metastasis was observed in the patients. Three women with mean age of 43 years (36-52) had local recurrence. Of these cases of local recurrence, two were in the proximal tibia and one in the distal radius.
Three men and two women with GCT of bone who underwent operative treatment at our hospital before March 2009 without local recurrence or pulmonary metastasis were enrolled in the study as a no-recurrence group (Table 1). Their mean age was 40 years (25-67). Of their primary lesions, two were in the distal femur, two in the proximal humerus, and one in the ischial tuberosity. Of the four GCT of bone, curettage without bone graft was performed in addition to one wide resection for the operative treatment. The mean follow-up period after the operation was 5 yr and 2 mo (3 yr, 4 mo - 9 yr, 5 mo).
The control group comprised patients who had been referred to our clinic presenting osteolytic lesions and for whom a histological diagnosis other than GCT of bone or chondroblastoma had been made (Table 2). These 8 men and 10 women had mean age of 62 years (32-79). Histological diagnoses were nine of primary and nine of metastatic malignant bone tumors. TRACP 5b was determined using commercial enzyme immunoassay (fragments absorbed immunocapture enzymatic assay, Osteolinks; DS Pharma Biochemical Co. Ltd.). Patients who had received bisphosphonates, selective estrogen receptor modulators (SERMs), or calcitonin therapy were excluded from the study. Patients who had undergone a fracture before operation or who showed hormonal abnormality were also excluded from the study. Student t-tests were used to compare the values of serum TRACP 5b, inferring p < 0.05 as significant.
Clinical Characteristics of the Control Patients
| Case No. | Age | Gender | Location | Diagnosis (Primary Lesion) | TRACP 5b |
|---|---|---|---|---|---|
| 1 | 56 | F | proximal tibia | clear cell sarcoma | 577 |
| 2 | 73 | F | calcaneus | chondrosarcoma | 408 |
| 3 | 32 | F | scapula | osteosarcoma | 158 |
| 4 | 70 | F | sternum | chondrosarcoma | 661 |
| 5 | 78 | M | femur | MFH of bone | 421 |
| 6 | 43 | F | pubis | chondrosarcoma | 419 |
| 7 | 60 | F | ilium | MFH of bone | 555 |
| 8 | 62 | F | ilium | chondrosarcoma | 405 |
| 9 | 56 | M | femur | MFH of bone | 408 |
| 10 | 64 | F | femur | metastasis (lung cancer) | 406 |
| 11 | 46 | M | femur | metastasis (lung cancer) | 364 |
| 12 | 79 | F | femur | metastasis (thyroid caancer) | 195 |
| 13 | 75 | M | multiple* | metastasis (gastric cancer) | 617 |
| 14 | 68 | M | acetabulum | metastasis (lung cancer) | 569 |
| 15 | 67 | M | humerus | metastasis (renal cell cancer) | 452 |
| 16 | 67 | M | pelvis | metastasis (renal cell cancer) | 328 |
| 17 | 78 | F | clavicle | metastasis (lung cancer) | 995 |
| 18 | 56 | M | pelvis | metastasis (thyroid cancer) | 467 |
* spine, pelvis, rib, etc.
RESULTS
TRACP 5b showed more than 1500 Um/dL with no relation to the tumor location or the size of osteolytic lesion in any patient of the primary group (Table 1). Mean TRACP 5b values decreased gradually at one week (783.8 ± 225.8, 526-1050 mU/dL) and at one month (501.3 ± 169.8, 274-760 mU/dL) after the operation and exhibited low values until local recurrence (Fig. 1). The TRACP 5b value increased gradually with development of local recurrence in a patient.

TRACP 5b values in the primary group. Each case number is the same as that shown in Table 1. TRACP 5b was measured immediately before the biopsy. The TRACP 5b value of case 1 increased gradually during 9 months after the curettage, implying local recurrence. However, the TRACP 5b value decreased dramatically after re-curettage. Case 3 showed a pathological fracture during curettage following bone union with no complication. Preop: before curettage.
Plain radiography also demonstrated that the progression of the osteolytic change and the lesion was enhanced extremely by Gd in MRI (Fig. 2). TRACP 5b decreased again immediately in the patient after a week of curettage (Fig. 1). The mean value of TRACP 5b of the local recurrence group was 753 ± 68.7 (656-856 mU/dL; Table 1).
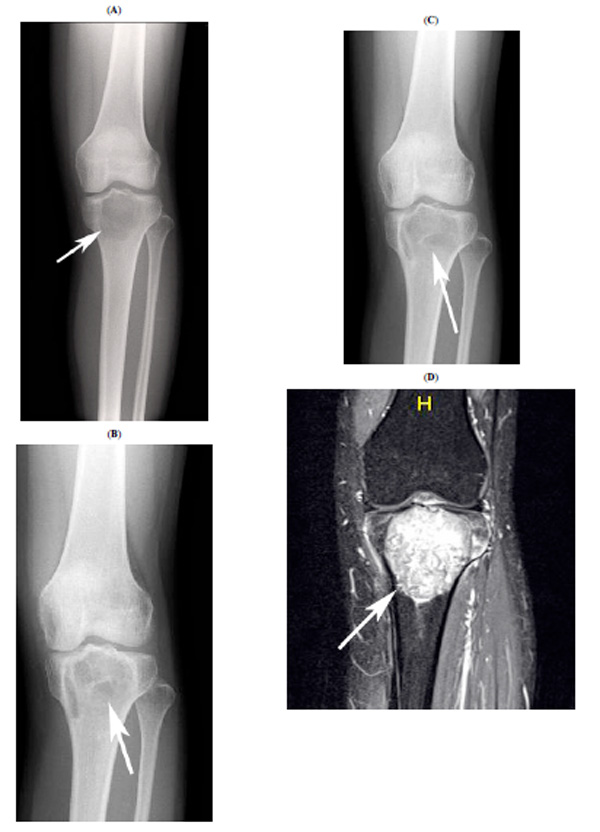
Radiographic findings for case 1. This preoperative plain radiograph shows the osteolytic lesion at the proximal tibia (A, white arrow). Bone formation is apparent at 8 months after curettage (B, white arrow). However, osteolytic change had progressed at 16 months after the curettage. A plain radiograph shows osteolytic lesions at 20 months after curettage (C, white arrow). The osteolytic lesion was enhanced extremely by Gd in MRI simultaneously (D, white arrow).
The time between the operation and the local recurrence did not affect the TRACP 5b value. However, TRACP 5b values increased gradually with the progression of local recurrence (Fig. 3). The osteolytic change also developed gradually on plain radiographs, as shown by case 5 (Fig. 4). In the no-recurrence group, the mean value of TRACP 5b was 340.6 ± 78.3 (168-493 mU/dL; Table 1).

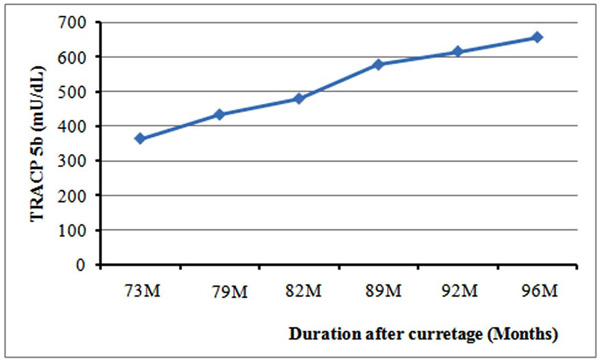
TRACP 5b values in the patient with local recurrence (case 5). When TRACP 5b was measured initially at 73 months after curettage, local recurrence was suspected from both clinical and MRI findings. The patient refused re-curettage for personal reasons. TRACP 5b values increased gradually thereafter.
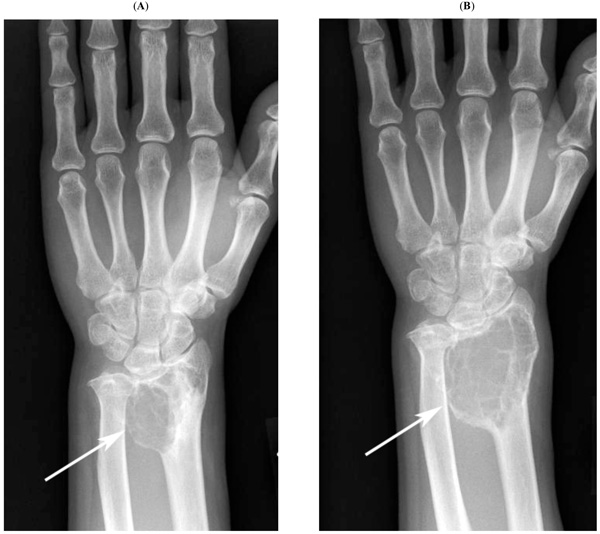
Plain radiographic findings for case 5. Plain radiography shows progressive osteolytic lesions (73 months (A) and 96 months (B) after curettage, arrows).
Statistically significant differences were found between the no-recurrence group and the local recurrence group (Fig. 5, p = 0.0017). Neither the follow-up period nor patient age showed any effect on the TRACP 5b value. The mean value of TRACP 5b of the control group was 466.9 ± 130.3 (158-995 mU/dL; Table 2). Little relation was observed between the TRACP 5b value and patient age. The mean value of the control group was much lower than that of the primary group. Moreover, it was significantly lower than that of the local recurrence group (Fig. 5, p = 0.006). However, no significant difference was found between the no-recurrence group and the control group (Fig. 5, p = 0.094).
Values of TRACP 5b in each group. Statistically significant differences were found between the no-recurrence group and the local recurrence group (*: p = 0.0017). Values of local recurrence were significantly higher than those of the control group (**: p = 0.006). However, no significant difference was found between the no-recurrence group and the control group (p = 0.094).
DISCUSSION
Although GCT of bone is locally aggressive, curettage is the most popular operative treatment for preserving the adjacent joint. Of all GCT of bone cases, 10-50% show recurrence following curettage [1, 2, 4-6, 18]. Some cases have multiple local recurrences. Repeat recurrence correlates with lung metastasis [6]. Histological grading described by Campanacci et al. [18] has been used for its prognosis [2], although histological grading does not correlate with local recurrence [6, 19]. To predict local recurrence, DMA cytometry [20], gene [21], and protein [22] analyses of the tumor are useful in addition to contrast-enhanced dynamic MRI [23, 24], but an important problem is that the genetic and biochemical methodologies used to detect those markers are complicated. Moreover, local recurrence is missed by MRI when no appropriate range of interest (ROI) is selected [23]. A new marker that can yield results easily is necessary to diagnose and to predict the local recurrence of GCT of bone. Serum total acid phosphatase was useful for the diagnosis and the detection of local recurrence [8-10]. However, it contains secretory acid phosphatase of various types including that from the osteoclasts [11]. Recently, serum TRACP 5b, a sensitive marker of bone resorption, was detected as secreted from osteoclasts [11, 25]. Osteoclast-like giant cells are prominent among the histological features of GCT of bone [1]. Based on these facts, serum TRACP 5b became a specific marker for GCT of bone, measured in patients with GCT of bone and other bone tumors presenting osteolytic lesions.
Serum TRACP 5b has been used for bone quality evaluation in patients with osteoporosis (normal range 170-590 mU/dL for men, 120-420 mU/dL for women). A commercial enzyme immunoassay kit for serum TRACP 5b was not applied initially to measure extremely high values such as 1500 mU/dL in patients with GCT of bone because the kit is used exclusively for measurement of serum TRACP 5b in patients with osteoporosis. However, more precise results have become obtainable recently. Serum TRACP 5b is present at much higher concentrations in patients with GCT of bone than in patients with local recurrence or some other bone tumor such as a primary malignant tumor and metastases. Statistical analyses were not performed because of the lack of precise values in the primary group. The apparent difference between the primary group and the other groups was found to be clinically significant. Neither tumor size, skeletal distribution of the tumor, nor patient age affected the serum TRACP 5b level. Similar results were obtained for the control group. Furthermore, serum TRACP 5b decreased dramatically at one week after curettage and remained at a similar level when no local recurrence was observed.
In these patients, plain radiography showed bone formation of the cured lesion. Little enhancement effect was observed in contrast-enhanced MRI. However, its level increased gradually with the progression of local recurrence. Plain radiography showed that osteolytic lesions developed gradually. Local enhancement became readily apparent in MRI compared to images obtained from cases without local recurrence [24]. Compared to radiological [24] or biomolecular [20-22] methods, serum TRACP 5b value is a more objective and smart specific strategy for use in cases of progression of GCT of bone.
An important limitation of the study is that the patients were few. However, these results need not be applicable for all forms of GCT. Some patients with GCT do not express a high level of serum TRACP 5b. Furthermore, heterogeneity of multinuclear or mononuclear cells in GCT affects the serum TRACP 5b level. Nevertheless, these results are noteworthy as a preliminary report: every patient with GCT in this study showed a similar tendency of the TRACP 5b value depending on the clinical course. Additional studies must be conducted to accumulate data for patients with GCT. Another limitation of this study is that patients with pulmonary metastases of giant cell tumor of bone are not included in this series. Fewer than 10% of the patients with giant cell tumor of bone develop lung metastases [1, 2, 4, 6, 18]. Results for local recurrence demonstrated that serum TRACP 5b can increase in patients with pulmonary metastases because of the similar histologies of primary and metastatic lesions. Another point of interest is how the serum TRACP 5b level is affected similarly by metastatic progression and by local recurrence.
Although the histological features of these tumors differ from those of GCT of bone, brown tumors, especially solitary lesions, and aneurysmal bone cysts show similar radiological findings to those of a giant cell tumor of bone. They are important for differential diagnosis [1, 26]. These lesions were not examined in the study, but serum TRACP 5b values of these tumors are interesting. Chondroblastomas, which also occur at the epiphyseal region of the long bone, show similar histology to that of GCT of bone. They are invariably associated with osteoclast-like giant cells [27]. Although the incidence of chondroblastomas is much less frequent than that of GCT of bone [1, 27], similar results can be expected because of their histological characteristics. Further studies must be undertaken to evaluate these possibilities.
In conclusion, serum TRACP 5b is a more useful and more convenient specific marker for the diagnosis and prediction of the recurrence of GCT of bone than methods and markers presented in previous reports describing MRI and DNA analysis.
ACKNOWLEDGEMENT
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.


