All published articles of this journal are available on ScienceDirect.
The Memory Metal Spinal System in a Posterior Lumbar Interbody Fusion (PLIF) Procedure: A Prospective, Non-Comparative Study to Evaluate the Safety and Performance
Abstract
Study Design:
A prospective, non-comparative study of 27 patients to evaluate the safety and performance of the Memory Metal Spinal System used in a PLIF procedure in the treatment of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease (DDD).
Objective:
To evaluate the clinical performance, radiological outcome and safety of the Memory Metal Spinal System, used in a PLIF procedure, in the treatment of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease in human subjects.
Summary of Background Data:
Spinal systems that are currently available for correction of spinal deformities or degeneration such as lumbar spondylosis or degenerative disc disease, use components manufactured from stainless steel or titanium and typically comprise two spinal rods with associated connection devices. The Memory Metal Spinal System consists of a single square spinal rod made from a nickel titanium alloy (Nitinol) used in conjunction with connection devices. Nitinol is characterized by its shape memory effect and is a more flexible material than either stainless steel or titanium. With current systems there is loss of achieved reposition due to the elastic properties of the spine. By using a memory metal in this new system the expectation was that this loss of reposition would be overcome due to the metal’s inherent shape memory properties. Furthermore, we expect a higher fusion rate because of the elastic properties of the memory metal.
Methods:
Twenty-seven subjects with primary diagnosis of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease (DDD) were treated with the Memory Metal Spinal System in conjunction with the Brantigan IF® Cage in two consecutive years. Clinical performance of the device was evaluated over 2 years using the Oswestry Disability Index (ODI), Short Form 36 questionnaire (SF-36) and pain visual analogue scale (VAS) scores. Safety was studied by collection of adverse events intra-operative and during the followup. Interbody fusion status was assessed using radiographs and a CT scan.
Results:
The mean pre-operative ODI score of 40.9 (±14.52) significantly improved to 17.7 (±16.76) at 24 months postoperative. Significant improvement in the physical component from the SF36 questionnaire was observed with increases from the baseline result of 42.4 to 72.7 at 24 months (p<.0001); The emotional component in the SF36 questionnaires mean scores highlighted a borderline significant increase from 56.5 to 81.7 at 24 months (p=0.0441). The average level of leg pain was reduced by more than 50% postoperation (VAS values reduced from 5.7 (±2.45) to 2.2 (±2.76) at 24 month post-operation with similar results observed for back pain. CT indicated interbody fusion rate was not significantly faster compared to other devices in literature. No device related adverse events were recorded in this study.
Conclusions:
The Memory Metal Spinal System, different from other devices on the market with regard to material and the one rod configuration, is safe and performed very well by improving clinically important outcomes in the treatment of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease. In addition the data compares favorably to that previously reported for other devices in the literature.
INTRODUCTION
Chronic low back pain can be the result of spondylolisthetic or degenerative lumbar segmental instability [1, 2]. Surgical treatment of this condition by fusion of the involved segments was introduced in the mid-1920s [1, 3]. Treatment of this condition is one of the most resources demanding in the Western world [4-7]. Indications of this operative technique, and outcome of this surgery are intensely debated [8-15]. The idea of lumbar or lumbosacral arthrodesis is to eliminate motion and thus to relieve pain [16]. King in 1944 [17] introduced internal spinal fixation in the lumbosacral region. The use of the pedicle for screw placement was introduced in 1969 [18], and efficient screw-rod connections have also been developed [19, 20].
Our goal was to develop a less rigid fixation device to enhance intervertebral fusion [21].
The Memory Metal Spinal System is a posterior system, consisting of Titanium pedicle screws and bridges and a single square memory metal rod. Spinal systems that are currently available use components manufactured from stainless steel or titanium. The spinal rod component used in this system is manufactured from Nitinol (NiTi), a nickel-titanium alloy. The characteristics of this alloy were first described by Buehler and Wang [22]. At present, the characteristics of this NiTi alloy are used clinically in wires for orthodontic tooth alignment, osteosynthesis staples, vena cava filters and other vascular applications [23-28]. The biocompatibility and safety of memory metal are discussed in literature [29, 30].
NiTi is a Memory Metal and is mainly characterized by its shape memory effect and its superelasticity. By using a memory metal in this new system the expectation was that there is a better maintenance of the reposition due to the metal’s inherent shape memory properties (continues reposition force) and enhancement of the fusion due to a less rigid system. Although correction in deformity (reposition) was not specifically tested in the current study, shape memory is an important property of memory metal and its potential in spinal correction has yet to be fully explored.
In this study the Memory Metal Spinal System was implanted in humans for the first time and used in conjunction with Brantigan IF® carbon fibre reinforced polymer fusion cage. Brantigan IF® cages have been used in the clinical setting for ten years with excellent outcomes reported at both 2 years [31,32] and 10 years post-surgery [33].
The main objectives of this study were to evaluate the fusion, the clinical performance, and safety of the Memory Metal Spinal System, used in a PLIF procedure, in the treatment of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease in humans.
MATERIALS AND METHODS
This was a multi-centre, prospective, non-comparative, post marketing surveillance (PMS) study to evaluate the Memory Metal Spinal System, used in conjunction with the Brantigan IF® Cage, in subjects who underwent a PLIF procedure.
Patients
Twenty-seven consecutive patients (9 male and 18 female) with a diagnosis of a symptomatic single level degenerative lumbar disc consented were treated with the Memory Metal Spinal System, used in conjunction with the Brantigan IF® Cage, following Research Ethics Committee approval. Inclusion criteria required all patients aged 18 years and over, with disabling back and/or refractory radicular pain who have had at least six weeks of conservative management, with moderate to severe degenerative changes in one or two lumbar disc levels based on MRI performed not more than three months prior to study entry. In addition discography had been provocative for patients back pain. Exclusion criteria ruled out patients with more than two abnormal lumbar disc levels, evidence of infection in the disc or spine, spinal tumor(s), who are immunocompromized, pregnant, and/or have a condition which would compromised their participation and follow-up in this study. Conservative treatment mostly entailed a combination of appropriate analgesics, physical therapy, and epidural and/or facet injections.
Implant Features
The Memory Metal Spinal System consists of pedicle screws, bridge connectors and one single square rode, made of a nickel-titanium alloy (Fig. 1). All the spinal rods used in the study were manufactured from medical grade nickel titanium alloy (also referred to as Nitinol or Shape Memory Alloy) according to ASTM F2063-00 standard. The rods have a 6.35mm square cross-section, similar to current systems and are available straight or pre-bent (pre-lordosed). The length of the rods can be cut to the specific length required by the surgeon by using a specially designed cutter. Standard pedicle screws were used (Monarch TMpedicle screw system, DePuy International). The transverse connector bridge is a device, which is fixed between two pedicle screws to produce a stable, rigid construct. The flexibility of the system comes from the rod. The rod is connected to the bridge and locked in place by a setscrew and sliding cap. The rod can be approximated to the bridge using a mini approximator instrument. All connection bridges are manufactured from medical grade Titanium Alloy that is considered safe to use with Nitinol.
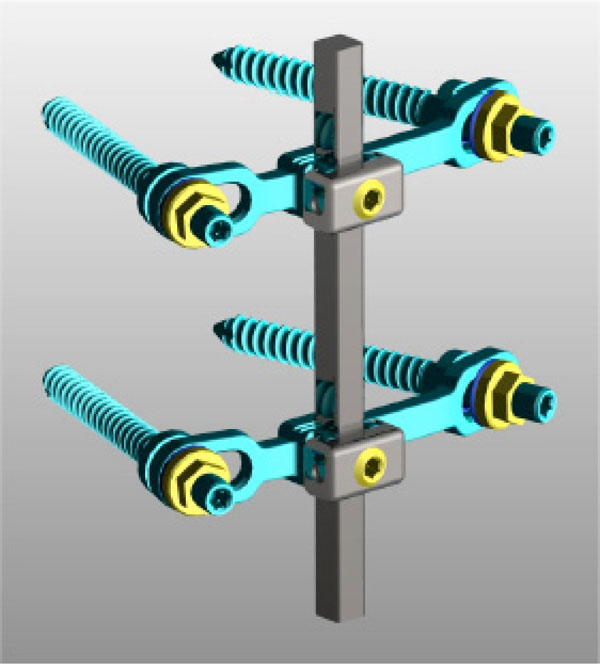
The single rod Memory Metal Spinal System.
Two experienced spine surgeons performed all surgeries in two consecutive years. A standard PLIF procedure was performed using the Memory Metal Spinal System (DePuy International) where after the Brantigan IF® Cage (DePuy International) was filled with autologous bone and placed in the intervertebral disc space.
Clinical and Radiological Outcome
Patients were evaluated pre-operatively at 1, 3, 6, 12 and 24 month after surgery. Evaluation at each interval included physical and neurological examination, concomitant medication, additional surgical procedures, subject completed questionnaires (Oswestry Disability Index, Short Form-36 Health) and Visual Analogue Scale for Pain. Any adverse events and complications were recorded.
Routine lateral and AP radiographs were obtained at each interval. Routine radiographs were used to evaluate the total intervertebral height and subsidence. The CT scan at two years follow-up was used to determine fusion. Interbody fusion was defined as complete bridging at any one or more points within the central area of the vertebral body as determined by CT. One independent radiologist who was not otherwise involved in the study determined intervertebral fusion assessments. Fusion was recorded as Yes/No/Can’t Assess.
Complications were divided into intra-operative and post-operative adverse events.
Statistics
For statistical analysis, comparisons between pre- and postoperative scores were made using paired t-tests.
RESULTS
Clinical Data
All 27 patients completed the 24 months of follow-up without any major adverse event. The average age of the patients was 44.3 (range 23.1-73.9). All patients had symptomatic single level degenerative lumbar disc (one patient L3-4, ten patients L4-5 and sixteen patients L5-S1).
The Oswestry Disability Index (ODI) functional outcome data compared baseline and post-operative results. The ODI mean score pre-operative was 40.9 (±14.52). This significantly improved to 17.7 (±16.76) at 24 months post-operative (p<.0001).
Significant improvement in the physical component from the SF36 questionnaire was observed with increases from the baseline result of 42.4 to 72.7 (±21.74) at 24 months (p<.0001); the emotional component in the SF36 questionnaires mean scores highlighted a borderline significant increase from 56.5 to 81.7 (±29.57) at 24 months (p=0.0441).
The average level of leg pain was reduced by more than 50% post-operation (VAS values reduced from 5.7 (±2.45) to 2.1 (±2.30) at 1 month post-operation). This reduction remained constant over the 24 months post-operation (2.2 (±2.76) at 24 month post-operation). A similar reduction in back pain was also revealed.
Bivariate analysis indicated that gender; previous non-surgical treatment, smoking history, and obesity had no statistical effect on clinical or fusion success.
Radiological Assessment
X-rays were performed on standing patients with both AP and lateral views being taken. The pre-operative and post-operative radiographic assessments were carried out on the patient population. A CT scan and X-rays were performed at 24 months post-operative. The data is illustrated in Fig. (2). An example of solid fusion on X-ray and CT is illustrated in Fig. (3a, b).
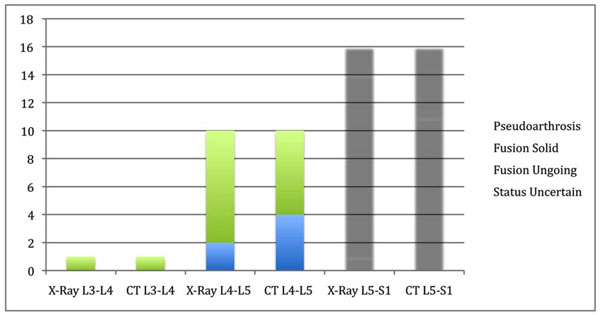
Fusion Assessment at 24 Months – X-Ray/CT L3-L4, L4-L5, L5-S1.

“Solid fusion” status (X-Ray).

“Solid fusion” status (CT).
Safety
During the 27 surgical implanting procedures, four intra-operative complications occurred. There was 1 (3.7%) malpositioning of a pedicle screw, 1 (3.7%) breakage of a cage and there were 2 (7.4%) dural tears. The implant breakage involved the inserted interbody fusion cage. The fracture was located around the threaded hole for the insertion tool and occurred on impaction into the disc space. The 2 dural tears were repaired during surgery, there was no neurologic injury and the hospital course was not affected. None of the adverse events were related to the device under investigation (i.e. biocompatibility issues).
DISCUSSION
Twenty-seven subjects with primary diagnosis of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease (DDD) were treated with the Memory Metal Spinal System in conjunction with the Brantigan IF® Cage. The mean pre-operative ODI score of 40.9 (±14.52) significantly improved to 17.7 (±16.76) at 24 months post-operative. Significant improvement in the physical component from the SF36 questionnaire was observed with increases from the baseline result of 42.4 to 72.7 at 24 months (p<.0001); The emotional component in the SF36 questionnaires mean scores highlighted a borderline significant increase from 56.5 to 81.7 at 24 months (p=0.0441). The average level of leg pain was reduced by more than 50% post-operation (VAS values reduced from 5.7 (±2.45) to 2.2 (±2.76) at 24 month post-operation with similar results observed for back pain.
The clinical outcome in this study is comparable to the literature. A study of 60 patients with posterior lumbar interbody fusion combined with instrumented postero-lateral fusion reported by Freeman et al. [36] indicated stable circumferential fixation as shown by radiographs and tomograms confirming the presence of a bridging fusion mass. Of the 48 ODI questionnaires completed after 5 years, 79% had an ODI <30. In the present study 74% (17/23) of the patients indicated an ODI < 30. McKenna et al. reported a prospective, randomized controlled trial of femoral ring allograft (FRA) versus a titanium cage (TC) in circumferential lumbar spinal fusion with minimum 2 years clinical results [37]. Comparison of change in ODI results indicated a significantly greater improvement in the FRA group (reduced from 57 to 42) when compared to the TC group (54 reduced to 48). The corresponding change in ODI results from baseline over 2 years in the current Memory Metal Spinal System study was greater than that of either the FRA or TC groups (23 versus 15 and 6). Both FRA and TC patients showed a significant improvement in VAS for back pain (change in VAS 1.9 and 1.1 respectively). However with leg pain VAS scores only FRA patients demonstrated a significant improvement (change in VAS of 1.3) whereas the TC group had more leg pain increasing the VAS scores postoperatively by 0.4 points. VAS results from the Memory Metal Spinal System study for both back and leg pain show a much greater improvement when compared to both groups in the FRA / TC study. Part of the explanation is that our patient group had significant more grade spondylolisthesis and therefore more leg pain. The review of Boos & Webb [38] suggests that PLIF in these cases do better than fusion alone. A study with two different patient groups of 30 subjects having spondylolisthesis, which were subjected to different surgeries: posterior lumbar fusion with pedicle screws (Group I) and posterior lumbar interbody fusion with pedicle screws (Group II) has also been reported [39]. The ODI mean scores pre-operatively and 2 years post-operatively were 28.5 and 18.6 respectively for Group I and 31.3 and 13.3 respectively for Group II. The ODI scores in the current study show a comparable result. Glassman et al. reviewed the ODI and SF36 outcomes in a multicentre lumbar fusion study with follow up after 2 years [40]. The minimal clinically important difference (MCID) seeks to differentiate a magnitude of change, which is not only statistically valid but also of real clinical value. Figures for MCID for ODI results have been reported as low as a 4 points decrease [41] and also a 10 points decrease [42]. The Food and Drug Administration (FDA) standards suggest a 15 point decrease in ODI and either maintenance of or any improvement in SF-36 Physical Composite Score (PCS) [43]. Ware et al. [44] reported that an increase of 5.42 points in the SF-36 PCS is clinically important. A more recent study [45] has reported the following MCID values: 12.8 points for ODI, 4.9 points for SF-36 PCS, 1.2 points for back pain and 1.6 points for leg pain. The improvement in ODI values for the various fusion treatments in the multicentre review ranged from 9.9 to 22.2 points whereas the improvement in SF-36 data ranged from 13.8 to 6.3 points. The improvement in the corresponding ODI and SF-36 values in the current Memory Metal Spinal System study were 23 and 31. The improvement in back and leg pain were 2.5 and 3.5 respectively. The results obtained for the Memory Metal Spinal System have therefore satisfied the MCID reported in the literature.
Radiological assessment indicated that interbody fusion rate was good after two years compared to other more traditional devices in literature. At 24 months post-operative there was a big difference in fusion status between X-Ray (91.0%) and CT (66.7%). These results are comparable to literature [34]. Most studies only use plain X-ray for fusion assessment that gives high false positive results. Shah et al. [35] showed that CT provides a more sensitive assessment of interbody fusion than plain radiographs, with a more robust inter-observer agreement comparable to our study results. The memory metal system in this study prove to be safe in comparison to the reports of West et al. [46] and Pihajamski [47] for pedicle srew studies, Hall et al. [48] for degenerative disc disease treated with the Isola pedicle screw system and Brantigan et al. [32] for PLIF procedure.
CONCLUSION
The Memory Metal Spinal System, different from other devices on the market with regard to material and the one-rod configuration, is safe and performed well (in regard to fusion) in the treatment of spondylolisthesis, symptomatic spinal stenosis or degenerative disc disease. The clinical outcome is comparable to traditional devices in the literature. The results of this study were used to support the further development of the 3-dimensional treatment system of scoliotic deformities.
CONFLICT OF INTEREST
Declared none.
ACKNOWLEDGEMENT
The study was sponsored by DePuy International.


