RESEARCH ARTICLE
Quantitative Assessment of Improvement with Single Corticosteroid Injection in Thumb CMC Joint Osteoarthritis?
Munir Khan*, Mohammad Waseem, Ali Raza, Damjana Derham
Article Information
Identifiers and Pagination:
Year: 2009Volume: 3
First Page: 48
Last Page: 51
Publisher ID: TOORTHJ-3-48
DOI: 10.2174/1874325000903010048
Article History:
Received Date: 27/4/2009Revision Received Date: 13/5/2009
Acceptance Date: 20/5/2009
Electronic publication date: 19/6/2009
Collection year: 2009

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
We present a unique prospective study to estimate the median sustained stage-related improvement in pain and hand function in patients with trapezio-metacarpal joint osteoarthritis after administration of a single corticosteroid injection. Response to the corticosteroid injection was assessed using Disability of Arm Shoulder and Hand (DASH) and Visual Analogue Scale (VAS) pain scores. Self assessment scores revealed a reduction in pain and improvement in hand function. All patients reported a significant improvement in pain and hand function (p ≤ 0.05), regardless of the disease stage. Additionally, we observed a marked difference in the duration of improvement in hand function between early and late stages of the disease (p = 0.0046). In conclusion, it is possible to predict the period of symptomatic improvement at each of the four radiological stages of the disease. This would allow the treating clinician to judge the outcome of treatment with reasonable accuracy.
INTRODUCTION
Carpometacarpal joint osteoarthritis at the base of thumb is a common problem in old age, particularly for women in the fifth to seventh decades. Estimated prevalence of the disease is 8% to 22% for women and 2% to 5% for men [1, 2]. Severe symptoms are often precipitated by a fall or strain on the wrist, following which the joint may never settle. Non-operative treatment entails splinting, NSAIDs and intra-articular injection of corticosteroids [3, 4], whereas surgical options include ligament reconstruction, excision arthroplasty and joint replacement [5-10]. Several guidelines recommend the use of intra-articular corticosteroids for the management of osteoarthritis. These drugs produce subjective improvement of symptoms in various anatomical regions [11-14] for a few weeks. But there is no consensus on their use, and no simple clinical predictors of response are available at present [15]. Thus, symptomatic relief following corticosteroid use depends on the biological history of individual patients. The use of intra-articular corticosteroids has been advocated in the basal thumb arthritis [5].
The aim of this study was to estimate the improvement in pain and hand function in patients who had a single intra-articular corticosteroid injection for thumb carpometacarpal joint (CMCJ) osteoarthritis.
MATERIALS AND METHODS
We recruited forty consecutive patients displaying trapezio-metacarpal arthritis who fulfilled the inclusion criteria and consented to take part in the study, from the outpatients clinics. None of the patients had been treated previously with a steroid injection into the joint. Informed consent was obtained from all the participants. All patients received 10mg of Kenalog (0.5 ml) and a local anaesthetic solution into the joint. Patients were grouped into various stages (I to IV), according to the radiological criteria [16], by three independent surgeons (Fig. 1). In case of dispute, a majority vote was counted. Inter-rater kappa values were calculated to determine consistency between individual surgeons’ X-Ray gradings. To establish whether patient groups based on the radiological stages were comparable, we compared gender, age and symptom duration of individuals between the four groups, using the Kruskal-Wallis test (Table 1). Patients were additionally assessed by a physiotherapist at six weeks after the injection.
Details of the Four Patient Groups. Values Represent Median and Inter-Quartile Range
| Group | Gender (M/F) | Age Mean and (IQR) | Mean Duration of Symptoms (in Months) |
|---|---|---|---|
| Stage I | 1/5 | 63.4(53-72) | 28 |
| Stage II | 5/13 | 64.2 (58-78) | 39 |
| Stage III | 3/7 | 66.2 (57-80) | 37 |
| Stage IV | 2/4 | 66.6 (59-81) | 41 |
| p value | 0.8 | 0.4 | 0.09 |
Outcome Measures
1. Subjective Improvement in the Hand Function and Pain
Response was assessed with the aid of the Disability of Arm Shoulder and Hand (DASH) [17] score and a visual analogue score prior to the injection, and six weeks and three to six months post-injection. During every visit, patients were requested to fill in a DASH score and a visual analogue score.
Statistical Analysis
At six weeks post-injection, improvement scores in hand function and pain were compared to pre-injection scores using the Wilcoxon signed-rank test for two related samples. Similarly, the DASH scores at six weeks were compared to three months and six months scores. Differences in improvement of hand function and pain at the early (I & II) and late (III & IV) stages of the disease were compared with the Mann-Whitney independent sample test. The DASH scores in Stage I and II patients were separately compared with those of Stage III and IV patients respectively, to overcome inaccuracies due to overlap between Stages II and III.
The Kaplan-Meier method was applied to estimate the median duration of sustained improvement at each stage of the disease, with 95% confidence intervals. The log-rank (Mantel-Cox) test was performed to establish whether patient groups categorised on the basis of radiological grade differed in terms of the duration of symptomatic improvement.
RESULTS
Forty patients were analysed, including 29 females and 11 males. Patient ages ranged from 53 to 81 years, with a mean age of 65.1 years. No patients were lost to follow-up. The symptom duration period ranged from 3 months to over five years, with a mean duration of 36 months. Six patients were diagnosed with stage I disease, eighteen with stage II disease, ten with stage III disease, and six with stage IV disease.
Radiological Grading of the Disease
There was moderate (weighted kappa: 0.58) to good (weighted kappa: 0.71) inter-rater agreement for the radiological grading of the disease.
Subjective Outcome (DASH and VAS Scores)
Pre-injection visual analogue and DASH scores are presented in Table 2. At six weeks, twenty-six patients displayed a DASH score improvement of at least ten points. Four patients showed an improvement of lower than ten points, while DASH scores deteriorated in ten patients. At six weeks, reduction in the pain (visual analogue score) was evident in all but two patients. Pre-injection visual analogue scores were significantly higher (median = 7) than those recorded at six weeks (median = 3) (T = 0, p ≤ 0.05, r = -0.82). Similarly, the DASH score at six weeks (median = 43) was a significant improvement in all patients, in relation to the pre-injection DASH score (median = 57.5) (T = 0, p ≤ 0.05, r = -0.72). A significant deterioration was noticed in DASH scores in all patients, at three months (median 46) and six months (median 52), as compared to six weeks stage (median 43) (p = <0.05) (Fig. 3).
Baseline Function (DASH) and Pain (VAS) Scores at Different Stages of the Disease. Values Represent Medians and Inter-Quartile Ranges
| Stage of the Disease | Pre-Injection DASH Scores | Pre-Injection VAS Scores |
|---|---|---|
| I | 44 (32-64) | 6 (5-7) |
| II | 52 (44-66) | 6 (5-8) |
| III | 66 (40-75) | 7 (6-8) |
| IV | 74 (54-91) | 8 (7-9) |
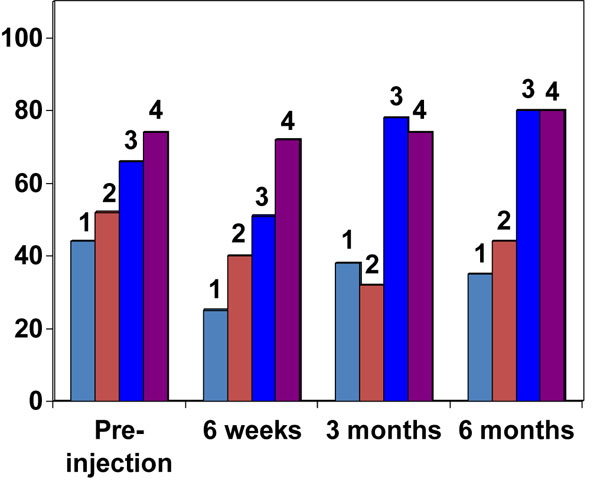 |
Fig. (3). DASH score in different radiological stages of the disease (Error bars show median & 95% Confidence intervals). |
Stage-related improvement in pain (VAS) is presented in Table 4. At six weeks, the visual analogue score improvement in patients diagnosed with early stages (I & II) of the disease (median = 3) was not significantly different from patients in later stages (III & IV) of the disease (median = 2), (U = 130, p = 0.08, r = -0.26). At six weeks the improvement in DASH scores in early-stage patients (median = 14.5) was comparable to those in late-stage patients (median = 8.5), (U = 127, p = 0.07, r = -0.28). Moreover, at six weeks, the DASH score improvement in Stage I disease patients (median = 14.5) was comparable to that in Stage III disease patients (median = 4.5) (U = 130, p = 0.09, r = -0.25). Similarly, the DASH score improvement in Stage II disease patients (median = 14.5) was analogous to that in Stage IV disease patients (median = 8.4) (U = 143, p = 0.08, r = 0.27).
Summary of the Changes in DASH AND Pain (VAS) Scores at Six Weeks, Compared to Pre-Injection Measures at Each Stage of the Disease. Values Represent Median and Inter-Quartile Range
| Stage of Disease | Improvement in DASH Score | Improvement in VAS Score |
|---|---|---|
| I | 14.5 (8-19) | 4.0 (2-5) |
| II | 14.5 (12-16) | 3.0 (3-7) |
| III | 4.5 (-6-15) | 2.5 (2-4) |
| IV | 8.4 (2-19) | 2.5 (0-6) |
However, at three months and six months (Fig. 3) the DASH score in the early-stage patients (median = 8) was significantly different from the late-stage patients (median = 1.5), (U = 50, p = <0.05, r = -0.61).
Duration of Symptomatic Improvement
Despite the comparable subjective improvement in hand function and pain between early and late stages of the disease at six weeks, there were significant differences (p = 0.0046) in the duration of symptomatic improvement (Table 3, Fig. 2). The duration of median sustained improvement in Stage I patients was 17 weeks. Most patients with stage II disease still reported improved hand function, at six months post-injection. In contrast, the reported median sustained improvement in Stage III and IV patients was only four weeks.
Median Duration of Symptomatic Improvement at Each Stage of the Disease
| Stage of the Disease | Median Duration of Symptomatic Improval (Weeks) | 95% CI |
|---|---|---|
| I | 17 | (13,21) |
| II | > 50% were still improved at 6 months. | - |
| III | 4 | (2,6) |
| IV | 4 | (2,6) |
 |
Fig. (2). Kaplan-Meier curve showing the duration of symptomatic improvement at each stage of the disease. |
DISCUSSION
This is a patient oriented study of the subjective improvement in pain and hand function following a single intra-articular injection of corticosteroid, for basal thumb arthritis. Our results showed that significant improvement in pain and hand function in patients with osteoarthritis of the thumb CMC joint can be achieved with a single corticosteroid injection. While the level of improvement achieved at six weeks was not related to the radiological stage, the duration of symptomatic improvement varied significantly, depending on the disease stage. This finding has two important implications. Firstly, corticosteroids improve pain and hand function, irrespective of the disease stage, and secondly, treatment with these drugs only provides long-term benefit at initial stages of the disease (I or II). At higher stages (III or IV), intra-articular corticosteroids may only be effective in patients awaiting definite surgical intervention. Furthermore, there was good inter-rater agreement for radiological staging of the disease using the Dell radiological criteria. Thus, we believe that our results can be easily reproduced in another clinical setting.
There are two limitations to our study. Firstly, we did not use fluoroscopy to ensure intra-articular delivery of corticosteroids. However, all injections were administered by an experienced physiotherapist. Moreover, the avoidance of fluoroscopy ensures that our results are more widely applicable, as fluoroscopy units are not generally available in outpatient departments. Secondly, relatively few patients with stage I disease were enrolled in the study, which may explain the unexpectedly short duration of symptomatic improvement, compared to stage II of the disease.
Day and colleagues [18] reported mean improvement for 23 months with a single intra-articular corticosteroid injection in stage I disease. Only 35% of patients with stage II and III disease displayed sustained improvement. Compared to their results, our stage I patients reported improvement for a shorter duration. However, it is important to note that patients in the earlier study wore splints, which made it difficult to estimate the improvement attributable to corticosteroid alone. Some patients in our study used splints, but were not requested to change their practice. We believe that this is a better way of estimating the effects of the intra-articular corticosteroid, as the patient’s baseline status is left undisturbed.
In conclusion, stage I disease patients had sustained symptomatic improvement for 17 weeks, although the true duration is possibly between 13 and 21 weeks. Most stage II patients still report improvement at 6 months post-injection. Stage III and IV patients had an identical prognosis of 4 weeks, although the true duration may be between 2 and 6 weeks. The findings of this unique study should allow the treating clinician to confidently predict the outcome of an intra-articular corticosteroid injection in patients presenting with thumb CMC joint osteoarthritis.
ACKNOWLEDGEMENT
The authors would like to thank Mr. Andy Biggs in photography department of The RJAH for producing illustration of the Dell's radiological criteria.








