SYSTEMATIC REVIEW
Use of Three-dimensional Printed Carpal Bones for Various Carpal Pathologies: A Systematic Review
Ishith Seth1, *, Gabriella Bulloch2, Nimish Seth4, Quentin Fogg2, David J. Hunter-Smith3, Warren M. Rozen3
Article Information
Identifiers and Pagination:
Year: 2023Volume: 17
E-location ID: e187432502306210
Publisher ID: e187432502306210
DOI: 10.2174/18743250-v17-e230711-2023-3
Article History:
Received Date: 20/02/2023Revision Received Date: 29/05/2023
Acceptance Date: 05/06/2023
Electronic publication date: 11/07/2023
Collection year: 2023

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution 4.0 International Public License (CC-BY 4.0), a copy of which is available at: https://creativecommons.org/licenses/by/4.0/legalcode. This license permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background:
Three-dimensional (3D) printing technology allows for patient-specific anatomical reconstruction. This study aims to summarize and critique the current literature on 3D-printed carpal bone implants used in various carpal pathologies.
Methods:
Web of Science, PubMed, Scopus, Google Scholar, and Cochrane Central Register of Controlled Trials databases were searched from January 1901 to October 2022. PRISMA guidelines were adhered to, and the study was registered on PROSPERO. Articles utilizing 3D printed carpal bone implants were selected based on pre-determined inclusion and exclusion criteria. The outcomes included intraoperative/postoperative complications, visual analogue score (VAS), disabilities of the arm, shoulder and hand (DASH) score, radial and ulna deviation. The Murad tool was used to assess the quality of case reports and Newcastle Ottawa scale was used to assess the observational studies.
Results:
A total of 6 studies comprising of 47 patients (34 males) were included. The average age was 35.3 years and indications for 3D printed implants included Fenton syndrome, Kienböck’s disease, and scaphoid non-union with and without necrosis. The overall postoperative VAS ranged from 0 to 1.4 and a significant reduction was noted from preoperatively with both rest and loading. The overall postoperative DASH score ranged from 9.2 to 25 and significant improvement was noted from preoperatively. The radial deviation ranged from 16.4° to 28.5° and while ulna deviation was from 23.8° to 36.4°. Only one complication was reported in included studies, a dislocation of the prosthesis. The overall quality of included studies was poor.
Conclusion:
3D-printed carpal bone implants improved outcomes in pain and function with minimal complications. The current study only reported only one complication postoperatively with no intraoperative complications. These results suggest that while 3D-printed carpal bone implants are still being optimized, large-scale clinical studies comparing the current options with the standard of care would provide better insights for recommendations and counseling.
1. INTRODUCTION
Carpal bone joint replacements are the cornerstone of treatment for end-stage degenerative and autoimmune-mediated bone disease [1]. Other operative treatments are currently more favorable as the pursuit of the ideal carpal bone implant is still ongoing. The common complications of carpal joint implant arthroplasty include dislocation, aseptic loosening, subluxation, and persistent pain resulting in high rates of revision and morbidity [2]. Therefore, with the emergence of three-dimensional (3D) technology allowing for ideal congruity with the restoration of normal anatomy may result in overcoming these limitations.
3D printing technology has gained significant popularity over the coming years to decades in many areas of medicine but specifically, orthopedics, where it has become commonplace for knee, hip, and ankle replacements [3, 4]. 3D-printed implants allow for patient-specific anatomical reconstruction and better surgical planning. These implants have highly organized microstructures which encourage resistance against compressive forces and overall reduce common complications such as subluxation, post-operative pain, long-term implant survival, and limited range of movement [5, 6].
The 3D-printed carpal bones have become curious topic for hand surgeons to contemplate, although a lack of industry interest and research has prevented the potential of carpal implants from being fully realized. While 3D knee implant arthroplasty has matured at a quick rate, 3D-printed carpal bones have lagged behind in comparison, carrying major barriers to their dissemination in clinical practice stem. A sec id="secal lack of information and research about the technology, the adoption of new techniques and cost of 3D printing currently prevent their full potential from being realised [7].
The leap to 3D implants could improve functional outcomes and complication rates of current carpal joint replacements, but a summary and critical address of the current literature is needed to steer future directions. Hence, the current study hopes to critique and summarize the current literature using 3D-printed implants for carpal bone implants, with attention to clinical and functional outcomes.
2. MATERIALS AND METHODS
This study was designed according to the Cochrane Handbook statement guidelines of systematic reviews and interventions and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [8]. The protocol was registered in the Prospective Register of Systematic Reviews, PROSPERO (CRD42022358748).
2.1. Search Strategy
Web of Science, PubMed, Scopus, Google Scholar, and Cochrane Central Register of Controlled trials (CENTRAL) databases were searched from January 1901 to October 2022. All references of eligible articles were retrieved and considered to identify possible missed citations. The following search term used in PubMed was (Three-dimensional OR Three dimension* OR 3D OR rapid) AND (print* OR prototyping OR implant* OR bone prostheses) AND (Osteoarthritis OR OA OR osteoarthrosis OR “degenerative joint disease” OR “degenerative arthritis” OR “revision Total joint Replacement*” OR “revision Total joint Arthroplast*” OR “revision total joint arthro*” OR “revision Total joint repair” OR “revision Total joint dislocation” OR “revision Total joint procedure” OR ” revision Total joint operation” OR ” revision Total joint surgery” OR DJD OR RTJR OR RTJA) AND (carpal OR scaphoid OR Lunate OR Triquetrum OR Pisiform OR Hamate OR Capitate OR Trapezoid OR Trapezium). Additionally MeSH terms included were (“Three-Dimensional Printing”[MeSH Terms] OR “3D Printing”[Title/Abstract]) AND (“Carpal Bones”[MeSH Terms] OR “Carpal Bone”[Title/Abstract]) AND (“Bone Implants”[MeSH Terms] OR “Bone Implants”[Title/Abstract] OR “3D-printed bone implants”[Title/Abstract]). The titles and abstracts of eligible studies were manually screened by two authors (IS and GB) based on relevance. Any disagreements were solved by a third reviewer (NS). The remaining full texts were retrieved and screened according to our eligibility criteria. Manual search of references for included studies was also performed.
2.2. Eligibility Criteria
The inclusion criteria for studies were as follows: 1) any study design - randomized controlled trial, nonrandomized prospective comparative, retrospective, case reports or case series; 2) use of 3D-printed carpal bones for any pathologies in human subjects.
The exclusion criteria were as follows: 1) animal models, cadaveric studies, review articles, pre-prints, conference proceedings, conference abstracts, and letters or editorial opinions; 2) studies not utilizing 3D-printed implants; 3) studies utilizing 3D printing guidance methods; 4) duplicated publications; 5) non-English studies.
2.3. Data Collection and Extraction
Titles and abstracts of studies identified during the search were imported into Endnote X9 (https://endote.com) for preliminary screening. Data extraction was carried out using an offline data extraction sheet after further checking to avoid any inclusion of data published in duplicates. Full texts of potentially relevant papers were further screened using the eligibility criteria by two independent reviewers (IS and GB), and any disparity in either selecting eligible studies or assessing findings was resolved through consultation with a third reviewer (NS).
The extracted data included the following: author's name, year of publication, intervention, sample size, country, age, gender of patients, follow-up duration, 3D-printed material, indication for 3D printing carpal bones, surgical technique, inclusion criteria, conclusion, and the outcomes including intraoperative and postoperative complications, postoperative visual analogue score (VAS) scores, postoperative disabilities of the arm, shoulder and hand (DASH) score, radial and ulnar deviation.
2.4. Methodological Quality Assessment
Two authors (IS and GB) independently assessed the quality of the included studies. The Murad tool [9] assessed the quality of the included case reports and the Newcastle Ottawa scale (NOS) assessed the quality of the other studies [10]. Any discrepancies between assessors were resolved through discussion and a third assessor (NS).
Murad tool consists of eight items divided into four domains: selection, ascertainment, causality and reporting. There were two ways for judgment that the tool developers suggested either summation of the scores of the eight binary responses or they preferred to judge the overall methodological quality based on the questions that sound important in each specific clinical scenario [9].
The NOS scale assessed the observational studies for specific methodological issues, which contributes to a study’s overall quality score. The scale gives a maximum score of 10 points, where the studies were categorized according to their score as follows: high risk of bias (0 – 3 points), moderate risk of bias (4 – 6 points), or low risk of bias (≥ 7 points).
3. RESULTS
3.1. Literature Search
A total of 173 potentially eligible records were extracted from the initial data retrieval process. After the removal of duplicates, 160 records were screened for title and abstract screening. Following, 15 articles were eligible for full-text screening and a total of 6 studies were included in the systematic review. After the references of these included studies were manually searched, no further articles were included. The study selection process is shown in Fig. (1).
3.2. Characteristics of Included Studies
The included six studies conducted in five countries comprising 47 patients, 34 males (72%) with an average age of 35.3 years [11-16]. Four studies [11, 12, 14, 15] used titanium as their 3D-printed material, and other studies used cobalt-chromium alloy, zirconia-alumina alloy, and polyethylene prosthesis [12, 16]. The patients in the studies included had various indications for 3D printed implants, including Fenton syndrome, Kienböck’s disease, and scaphoid non-union with and without necrosis. The included studies characteristics are shown in Table 1.
3.3. Quality Assessment
Most of the included studies had adequate follow-up periods to evaluate their long-term outcomes, but the overall quality of case reports/series and observational studies were poor. The risk of bias assessment is shown in Tables S1 and S2.
3.4. Outcomes
3.4.1. VAS Score
VAS scores compare pain on a scale from 0-10 with higher numbers corresponding to more significant pain and were reported in five of the included studies [11, 13-16]. VAS scores ranged from no pain in Sánchez et al. [13] to 2 in Xie et al. [16] The study by Cioffi et al. [11] reported a VAS score of 1.7 in the partial 3D scaphoid printed group, and 1.4 in the complete 3D scaphoid printed group. Ma et al. and Rossello et al. reported VAS scores of 0.2 and 1, respectively [14, 15].
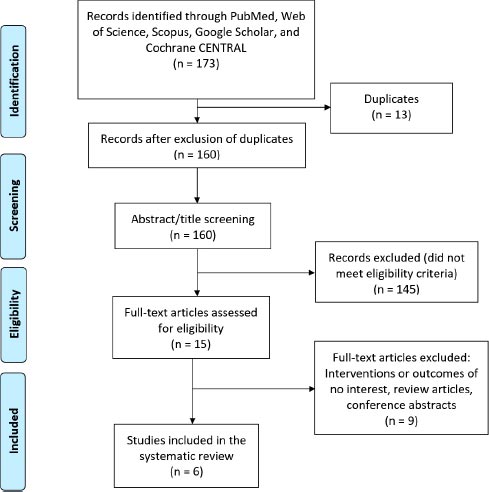 |
Fig. (1). PRISMA study selection process of included studies. |
| Study | Intervention | Study sample | Site | Age (mean±SD) | Male, n (%) | Follow up duration (mean±SD, months) | 3D printed material | Indication for 3D printing carpal bones | Surgical technique | Intraoperative and postoperative complications | Postoperative VAS scores | Postoperative DASH scores | Last visit radial deviation, (mean; degrees) |
Last visit ulnar deviation, (Mean; degrees) |
Inclusion criteria | Main outcomes | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cioffi et al. 2021 | Partial scaphoid 3D printed prothesis | 9 | Brazil | 27.5 ± 5.48 | 9 (100) | 16.5 ± 1.93 | Titanium | Scaphoid non-union with necrosis | Scaphoid arthroplasty | 0 and postoperative prosthesis dislocation requiring removal of implant and 3-corner arthrodesis | 1.7 | 9.2 | 26.45 ° | 36.42° | 1. Patients had a proximal pole of scaphoid non-union with necrosis 2. Failed the conservative or classical treatment 3. Submitted to preoperative MRI and TC |
1. Change in radioscaphoid angle 2. Flexion and extension of the wrist 3. Radial and ulnar deviation 4. VAS and DASH scores |
“The study is unique even if preliminary. The use of these devices can mark new borders of scaphoid fracture treatments. Considering this we believe that any scaphoid prosthesis and any biological scaphoid reconstruction must replicate the original shape of the bone as precisely as possible to minimize non-physiological kinematics and wear. This concept requires a patient-specific implant” |
| Total scaphoid 3D printed prothesis | 10 | 33.3 ± 4.33 | 8 (80) | Scaphoid non-union | Scaphoid arthroplasty | 0 | 1.4 | 11.8 | 28.5 ° | 35.5° | |||||||
| Ghali et al. 2021 | 3D printed lunate reconstruction | 20 | Morocco | 43 ± 11 | 13 (65) | 30 (mean) | 1. Titanium (Ti-6Al-4V) 2.Cobalt/Chromium Alloy 3. Zirconia/Alumina Alloy |
Kienböck’s disease or osteoarthritis of the lunate bone of the hand | Lunate arthroplasty | 0 | NR | NR | NR | NR | 1. The presence of Kienböck’s’s disease or osteoarthritis of the wrist 2. No exclusion criteria related to age or gender |
Lunate fractures measurements | “Finally, we proceed to the 3D printing of a prototype. The results obtained are satisfactory and could contribute to a better management of patients suffering from Kienböck’s’s disease” |
| Sánchez et al. 2020 | Scaphoid 3D-printed prothesis | 17y old male | Brazil | 17y | 1 (100) | 12 | Acrylonitrile-butadiene-styrene | Fenton syndrome | Scaphoid and capitate arthroplasty | NR | No pain at last follow up | 25 | 25° | 30° | 1. A 17-year-old male 2. Falling from a bicycle and sustaining direct trauma to the left wrist 3. With hyperextension and axial compression |
1. Dorsal and palmer flexion 2. Radial deviation 3. Wrist, claw, and grip functions 4. DASH scores |
“Cutting-edge diagnostic technologies, including three-dimensional printed models, are becoming essential tools, enabling the treatment of complex transscaphocapitate fracture-dislocations using open reduction and internal fixation with screws, with excellent outcomes at a 12-month follow-up period” |
| Rossello et al. 2020 | Scaphoid 3D-printed prothesis | A 34y old male | Italy | 34y | 1 (100) | 12 | Titanium | Scaphoid non-union with necrosis | Scaphoid arthroplasty | NR | 1 | 13.3 | 30° | 35° | 1. A 34-year-old male 2. Experienced wrist trauma three years ago 3. The patient ignored the injury until severe pain and functional loss |
1. Range of motion 2. Wrist, claw and grip functions 3. VAS and DASH scores |
“At 1-year follow-up, good clinical and radiographic outcome was obtained. Titanium custom-made 3D-printed implants may offer a good surgical solution for patients requiring total scaphoid replacement” |
| Zhen-jiang Ma et al. 2020 | 3D-Printed Lunate prothesis | 5 | China | 51.05 ± 7.8 | 2(40) | 20.7 ± 6.39 | Titanium alloy (Ti-6Al-4V) | Kienböck’s Disease | Lunate arthroplasty | 0 | 0.2 | 10 | NR | NR | 1. Continued pain after conservative care 2. Incapability to fulfill employment because of wrist symptoms 3. Unwillingness to pursue further conservative care 4. Absence of surgical CIs |
1. VAS and DASH scores 2. The active movement of wrist and strength |
“For patients suffering advanced Kienböck’s disease, lunate excision followed by 3D printing prosthetic arthroplasty can reconstruct the anatomical structure of the carpal tunnel, alleviate pain, and improve wrist movement” |
| Xie et al. 2017 | 3D printing lunate prosthesis | A 41-year-old female | China | 41y | 0 | 24 | Polyethylene | Stage IIIc Kienböck’s disease | Lunate arthroplasty | NR | 2 | NR | 16.4° | 23.8° | 1. A 41-year-old female 2. Of wrist pain for more than 2y after an accident 3. Felt wrist swelling, chronic pain, fatigue, limited activity 4. Unable to adhere to heavy manual jobs |
1. Dorsal and palmer flexion 2. Radial deviation 3. Wrist, claw and grip functions 4. Range of motion 5. VAS and coonery scores |
“We demonstrated that an anatomical reconstruction of Kienböck’s Disease is possible using 3D printing lunate prosthesis” |
Comparing VAS scores postoperative to preoperatively, Ma et al. reported a significant decrease in VAS from 7.3 to 0.2 and Rosello et al. [15] reported a decrease in VAS score from 3 to 0 at rest and 6 to 1 with the load. Lastly, Cioffi et al. [11] reported a decrease in VAS from 3.6 to 0 at rest and 7.4 to 1.7 under load.
3.4.2. DASH Score
DASH score ranges from 0-100 and a higher dash score represents greater functional impairment [17]. DASH score was reported in four studies [11, 13-15] and ranged from 9.2 in the partial 3D scaphoid printed group of Cioffi et al. to 25 in Sánchez et al. [11, 13]. The total 3D scaphoid implant by Cioffi et al. reported a score of 11.8, while Ma et al. and Rossello et al. had scores of 10 and 13.3, respectively [11, 14, 15].
The included studies postoperative scores reported a significant decline in DASH scores (functional improvement) compared to preoperative status. Cioffi et al. [11] reported 59% decrease in DASH score at 1 year to follow-up. While Rosello et al. [15] reported 70% decrease in DASH score from preoperative baseline score.
3.4.3. Radial Deviation
Four studies [11, 13, 15, 16] investigated radial deviation at follow-up. The mean deviation ranged from 16.4 degrees(°) by Xie et al. to 28.5° in the total 3D scaphoid group by Cioffi et al. [11, 16]. The partial 3D scaphoid printed in Cioffi et al. reported a mean deviation of 26.45°, while Sánchez et al. and Rossello et al. had mean deviations of 25° and 30°, respectively [11, 13, 15].
3.4.4. Ulnar Deviation
Ulnar deviation at follow-up was investigated in four studies [11, 13, 15, 16]. The mean deviation ranged from 23.8° in Xie et al. to 36.42° in the partial 3D scaphoid printed group by Cioffi et al. [11, 16]. The total 3D scaphoid printed group by Cioffi et al. reported a mean deviation of 35.5°, and Sánchez et al. and Rossello et al. had mean deviations of 30° and 35°, respectively [11, 13, 15].
3.4.5. Complications
One complication was reported in included studies [11]. Cioffi et al. reported one case of prosthesis dislocation at 1 month requiring the removal of the implant and performing 3-corner arthrodesis [11]. There were no reports of infection, cyst formation, and synovitis [14]. Furthermore, no postoperative complications were reported by Ghali et al. of investigating the implant by radiological assessment [12].
4. DISCUSSION
This systematic review investigated six studies for their indications and outcomes following 3D-printed carpal bone implants. The indications for 3D-printed implants included Kienböck’s disease, Fenton syndrome, and scaphoid non-union. Overall, 3D-implants reported DASH and VAS score improvement and significant improvement in ulnar and radial deviation. Although the literature for 3D-printed carpal bone implants is still in its infancy, the findings from this study indicate these resulted in improved outcomes and advocates for future large-scale clinical studies.
The adoption of 3D-printed implants lends to greater care in preoperative planning and improved implant compatibility, which may remedy the currently high intraoperative and postoperative complications associated with traditional total carpal replacements. Overall, this greater capacity to individualize and plan may be an underlying factor that conferred the good postoperative VAS and DASH scores, low postoperative complication rates and no recorded intraoperative complications and only one postoperative complication observed in the current review [11, 13-16]. Future studies would benefit from also assessing carpal joint range of motion, estimated blood loss, operation times and patient reported satisfaction, which may provide better evidence to suggest these benefits may be related to preoperative planning. It should be mentioned 3D implants allow for ligament restoration, unlike the biocompatibility of silicone and titanium implants in the majority of the studies is associated with postoperative inflammation, indicating material sensitivity to surrounding tissues [15]. Considering the prosthetic instability and silicone-induced synovitis from the Swanson silicone implants, 3D carpal implants may make headway for better patient outcomes and satisfaction [15, 18-21]. Although 3D implants hold great potential, approximately three weeks are required for their production [20] and the cost of production is high [15]. Additional studies comparing different 3D-printed materials with standard-of-care implants should be explored to investigate whether they may remedy the limitations of current carpal implant arthroplasties.
Traditional implants are pre-made and intraoperatively fitted to the patient, which largely ignores an individual’s unique anatomical variances, whilst 3D printing personalizes an implant with the aim to restore functional anatomy. As 3D implants are tailored for the individual, this approach minimizes the likelihood of distorting surrounding connective tissue and other anatomical structures, which would otherwise interfere with long-term outcomes, including pain, range of movement and subluxation [22]. For example, Xie et al. applied the principle of mirrored symmetry between the diseased and healthy bones to restore the lunate in an individual with advanced Kienböck's disease (stage IIIc) whereby 3D scanning matched bony surfaces on the healthy bone which was eroded otherwise on the diseased bone. Throughout recovery, the patient's wrist had no signs of weakness and regained near complete range of motion, even when participating in sports [16]. Through a similar approach, Ma et al. demonstrated their 3D-printed lunates resulted in greater VAS and DASH scores compared to scaphocapitate fusion and wrist arthrodesis, proximal row carpectomy or radial osteotomy [23-28]. These results suggest 3D-printed lunate may achieve a greater reduction in postoperative VAS compared to scaphocapitate fusion and wrist arthrodesis, proximal row carpectomy or radial osteotomy for Kienböck's disease, with VAS ranging from 1-4 in carpectomy, 1.6-3.4 in radial osteotomy, DASH score ranging from 0-18 in radial osteotomy and 14-32 in wrist arthrodesis [23-28]. Considering no procedure consistently provides good pain relief and functional outcomes for Kienböck's disease, the current literature suggests 3D-printed lunate implants should be considered alongside standard-of-care treatments in future clinical trials.
3D printing technology has been adopted for the creation of customized hip and knee joint replacement, allowing for personalization of individual anatomy and better outcomes. Although knee implants require fundamentally different operations and techniques to carpal bone implants, 3D-printed technologies provide improvements in pre-operative planning which may confer similar benefits across implant type. For 3D-printed knee implants, studies continually report improved post-operative VAS score, ROM, functional outcomes [3, 29], lower operative time and blood loss. Like the current study, Zhang et al. [29] included six studies and investigated 3D-printed total hip and knee arthroplasty with significant improvements for functional, clinical, and patient reported outcomes compared with routine prosthesis [30-34]. The current systematic review is unable to provide statistically significant findings, however, it seems carpal bones may provide similar outcomes to 3D-printed knee implants.
The current study highlights the potential of 3D-printed carpal implants for the treatment of various carpal pathologies. However, some limitations should be acknowledged. First, due to the paucity of case-controlled studies, a definitive clinical recommendation through a meta-analysis was not possible. Secondly, the included studies were of poor quality with three of the six included studies being case reports and this limited the reliability of these statements and questioned the integrity of previous research. Thirdly, as 3D-printed implants are costlier than routine surgery, a cost analysis would be beneficial however, this was not possible with the current information. Currently, 3D printing carpal bone implants cost higher than traditional implants due to the added cost of 3D printing process and materials, however, advancements in 3D printing technologies should reduce the cost over time. Future studies should perform a cost analysis between traditional and 3D printed implants, which would assist patients and insurance companies to make informed decisions. Future studies ought to include extended radiological follow-ups to assess the persistent alignment of these prostheses over time, particularly given the high rate of subluxation and dislocation associated with traditional carpal prostheses. Nevertheless, outcomes from the included studies hold anecdotal promise for improvements in pain, function, and ulnar/radial deviation. Lastly, the current study is the first to summarize the potential of 3D-printed carpal bone implants for carpal pathologies but calls for large-scale prospective studies to validate the current findings and address these limitations.
CONCLUSION
This systematic review found 3D-printed carpal bone implants improved outcomes in pain and function with minimal complications. These results infer that while 3D-printed carpal implants are still in their infancy, they have potential applications to address the limitations of current joint replacement options. Large-scale clinical studies comparing them with current standard of care are needed to confidently state the usefulness of 3D implants for carpal bone reconstruction.
LIST OF ABBREVIATIONS
| 3D | = Three-dimensional |
| DASH | = Disabilities of Arm, Shoulder and Hand |
| NR | = Not Reported |
| VAS | = Visual Analogue Scale |
| SD | = Standard Deviation |
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines and methodology were followed.
AVAILABILITY OF DATA AND MATERIAL
The data that support the findings of this study are available from the corresponding author [I.S] upon reasonable request.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.
SUPPLEMENTARY MATERIALS
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.








