All published articles of this journal are available on ScienceDirect.
Initial Perioperative, Work Status, Activity and Safety Outcomes after Decompression and Dynamic Sagittal Tether Stabilization versus Transforaminal Lumbar Interbody Fusion for Degenerative Spondylolisthesis: Interim Results from an FDA IDE Trial.
Abstract
Background:
Symptomatic lumbar degenerative spondylolisthesis (DS) is most commonly treated with decompression and fusion to address both the neurologic symptoms and underlying instability. However, fusion has known drawbacks, including invasiveness, recovery time and cost. A novel dynamic sagittal tether (DST) was developed to provide anatomic segmental stabilization after decompression by augmenting the posterior tension band.
Objective:
The objective of this study was to evaluate perioperative, work status and activity outcomes of decompression and DST stabilization (D + DST) vs. decompression and fusion (D + TLIF) from an ongoing FDA IDE study.
Methods:
Preoperative through 3-month outcomes and safety data from the IDE study (NCT03115983) are presented here. All patients had symptomatic Grade I DS with spinal stenosis, preoperative ODI≥35 and VAS leg/hip pain≥50. A propensity score (PS) model was utilized to control for inter-group differences in this parallel assignment (non-randomized) study. One hundred forty (140) patients had D + DST and 147 had D + TLIF. Perioperative characteristics, patient-reported outcomes, work status and activities of daily living (ADL) were analyzed with propensity score PS-adjusted difference and confidence intervals or chi-squared tests for multiple categorical variables. Kaplan-Meier survivorship analyses were performed for return-to-work and ADLs.
Results:
There were no significant PS-adjusted demographic, functional, disease or radiographic characteristic differences between groups preoperatively. The D + DST group had a PS-adjusted mean 70-minute shorter operative time, 183-ml less estimated blood loss and 2.3-day shorter hospital stay, with 66% of D + DST patients discharged the day of surgery and 88% discharged by the first postoperative day. At both the 6-week and 3-month intervals, more D + DST patients reported returning to work and ADLs. Both groups experienced significant reductions in leg/hip and back pain as well as disability 3-months postoperatively, while the D + DST group had significantly lower disability scores 6-weeks postoperatively. There were no significant differences in safety outcomes between the two groups, with 29 serious adverse events (SAEs) and 2 secondary surgeries (1.5%) in the D + DST group vs. 28 SAEs and 3 secondary surgeries (2.1%) in the D + TLIF group.
Conclusion:
Compared to D + TLIF, the D + DST procedure was shorter, less invasive and had a faster discharge. Faster recovery, return to work and ADLs with lower disability at 6 weeks were noted in the D + DST group. If longer-term results are durable, the DST may represent a less invasive stabilization alternative after decompression compared to instrumented fusion.
Trial Registration Number: NCT03115983
1. INTRODUCTION
Degenerative spondylolisthesis (DS) was first described by MacNab as a spondylolisthesis with an intact neural arch caused by sagittal orientation of the degenerative, arthritic facet joints, resulting in increased anterior vertebral displacement in flexion [1]. The neurologic symptoms of lumbar spinal stenosis (LSS) secondary to DS may be initially resolved with surgical decompression. However, several studies have shown that decompression alone may lead to progressive instability and less durable clinical outcomes [2-5]. This has led to decompression and instrumented fusion becoming the dominant procedure for this condition, however the cost, invasiveness and complications associated with lumbar fusion are clear and higher than decompression alone. There are proposed classification systems for DS that may identify which patients require fusion [3, 6-8], however, there are also studies that have shown no benefit of adding fusion to decompression for symptomatic DS in certain populations [9, 10]. This ongoing debate implies a treatment gap between surgical decompression and fusion for symptomatic DS.
Previous motion-preserving implants have not addressed this gap. Lumbar disc replacement is typically contraindicated for patients with DS due to facet joint arthropathy, certain radiculopathies or instabilities and osteopenia/osteoporosis [11, 12]. Interspinous spacers intended for use without a decompression have had poor outcomes in DS [13], and interspinous/interlaminar devices designed to be used with surgical decompression are indicated only for stable DS or as an adjunct to interbody fusion [14, 15]. Interspinous or interlaminar devices induce segmental flexion or kyphosis and may put the facet joints into an unstable posture with less joint surface overlap that further propagates the flexion-translation instability first described by MacNab and further shown radiographically by Toyone et al. [1, 16]
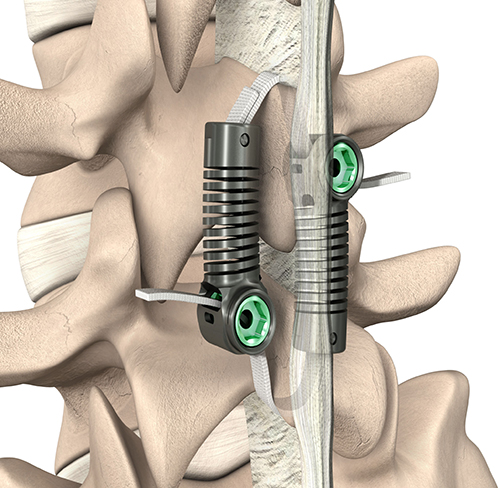
The objective for the treatment of symptomatic DS should be to durably resolve the patient’s symptoms with the least disruptive procedure. To this end, a dynamic sagittal tether (DST, Fig. 1) has been developed to be used with a direct lumbar decompression as an alternative to decompression and fusion for DS. DS involves flexion-translation instability that is further exacerbated by the decompression, which always involves removing structures posterior to the center of rotation, decreasing the resistance to flexion. The DST is designed to restore segmental flexion-bending stiffness, maintaining the segment in a relatively more lordotic posture such that the facet joints are engaged to reduce the coupled flexion-translation described by MacNab and Toyone et al. [1, 16, 17] The DST may offer the durability of fusion to maintain the effectiveness of the surgical decompression, while avoiding many of the associated complications and costs.
Spinal surgery generally has a 90-day “global” period covering the procedure and postoperative visits [18]. This interim study analysis aims to assess procedural characteristics, safety and recovery, including return to work and activities of daily living from the respective procedures through the first postoperative 90 days or 3 months.
2. METHODS
2.1. Study Design and Analysis Set
This study represents interim results of an ongoing FDA investigational device exemption (IDE) pivotal study (NCT03115983) comparing decompression and DST stabilization (D + DST) and decompression and transforaminal lumbar interbody fusion (D + TLIF) for symptomatic lumbar degenerative spondylolisthesis. The parallel assignment (non-randomized) design of the IDE trial assigned sites to exclusively enroll either into the D + DST or D + TLIF group. The D + DST group was enrolled prospectively, while the D + TLIF group included a prospectively enrolled cohort as well as retrospectively enrolled patients, provided that patient charts included sufficient information to verify eligibility and required outcomes. Twenty-seven sites participated, with approval and oversight from the governing institutional review boards.
Patients with Meyerding Grade I DS (10-25% anterolisthesis in an upright lateral X-ray) with at least moderate LSS requiring decompression at one lumbar level (L1-S1), ODI ≥ 35/100, VAS leg/hip pain ≥ 50/100 and no prior surgery at the index level were included in the study. Only prior decompression or discectomy were allowed at non-index lumbar levels. Patients were allowed to have additional decompression at one adjacent level. Full eligibility criteria are described at clinicaltrials.gov [19]. This analysis considers perioperative through 3-month outcomes from the IDE trial to assess procedural characteristics, safety and recovery from the respective procedures. Institutional study funding was provided by the manufacturer of the DST (Empirical Spine, San Carlos, CA). The DST is an investigational device and is not yet approved or cleared by FDA or other regulatory bodies for the indication described in this work.
2.2. Surgical Procedures
2.2.1. Investigational Procedure: Decompression and DST Implantation
Patients assigned to the D + DST arm received surgical decompression at up to two adjacent levels as indicated radiographically and clinically. Decompression was performed according to the treating surgeon’s preferred technique. The segmental stenosis was required to be amenable to a direct surgical decompression. Because the DST is secured to the spinous processes, the decompression was required to maintain at least half of each spinous process at the instrumented level [20]. At least 50% of the facets were also required to be estimated by the surgeon to remain after the decompression to ensure sufficient articular engagement. Following decompression, the patient’s position was confirmed or adjusted on the operative table into a lordotic posture, simulating the neutral standing alignment. The DST was then implanted at the DS level using specialized instruments to pass the bands around the spinous processes (piercing the adjacent interspinous ligaments), position, tension and lock the implant (Fig. 2). Fluoroscopy was used to verify the final implant position and tensioning. The excess band was cut off with a blade, and the incision was closed with standard techniques. An animated rendering of the DST implantation procedure is provided in the supplementary materials.
2.2.2. Control Procedure: Decompression and TLIF
Patients assigned to the control arm received decompression at one or two adjacent levels and TLIF at the single level of DS. The TLIF included an interbody cage and concomitant posterolateral fusion (PLF) with titanium rods and top-loading polyaxial screws. Allowed graft materials included autograft and allograft, as well as synthetic extenders, provided they were used on-label.
2.3. Outcome Measures
Demographic information, visual-analog scale (VAS), back/hip and leg pain, Oswestry disability index (ODI) and Zurich claudication questionnaire (ZCQ) were collected preoperatively. Radiographic parameters measured preoperatively included angular motion and translation in flexion/extension, anterolisthesis, sagittal disc angle and disc height and coronal facet joint angle, as analyzed by an independent radiographic core laboratory (Medical Metrics, Houston, TX). Postoperative evaluations at 6 weeks and 3 months included neurologic examination (motor and sensory), VAS leg/hip and back pain, ODI, and ZCQ. At each follow-up visit, patients were asked to report whether and when they had returned to work and normal activities of daily living (ADL). Adverse events and reoperations were tracked throughout the postoperative period.
2.4. Statistical Analysis
Due to the parallel assignment design of the IDE trial, a propensity score (PS) model was developed to control for selection bias and appropriately compare between-group differences, as utilized for other recent spinal IDE trials [21, 22]. Twenty-five preoperative covariates were included in the PS model, including demographics, disease characteristics, medical history, work status and radiographic measures. Radiographic measures of translation, disc height and facet joint angle were specifically included due to the findings of Blumenthal et al that these parameters influence surgical outcomes of patients with DS treated with decompression without fusion [3]. Covariate balance was assessed via the PS-adjusted standardized mean difference of the covariates [23, 24]. The primary endpoint of the IDE trial is detailed at clinicaltrials.gov [19]. This interim outcomes report documents perioperative outcomes through 3-month follow-up as a comparative characterization of the two procedures with no pre-defined primary outcome specified for this interim analysis. The IDE trial enrollment sample size was determined to ensure that the primary endpoint of the IDE study would be sufficiently powered, with success estimates based on previously reported outcomes for the DST and fusion treatments [14, 25]. Outcomes are presented as summary statistics, with between-group comparisons presented as PS-adjusted differences and 95% confidence intervals (CI). Between-group differences of multiple-categorical variables were assessed using chi-squared tests with a significance value of p = 0.05.
3. RESULTS
3.1. Demographics
Of a total of 299 subjects enrolled in the trial, 287 subjects were selected in the PS model: all 140 D + DST and 147/159 (92%) D + TLIF. All D + DST patients were enrolled prospectively, while in the D + TLIF control group 108/147 patients (73%) were enrolled prospectively and 39/147 patients (27%) were enrolled retrospectively.
Demographics of the PS-selected subjects are summarized in Table 1. The chi-squared test identified different racial distributions between groups (more Asian D + DST subjects vs more Black D + TLIF subjects), however the PS-adjusted differences demonstrated no significant differences between groups in any other preoperative demographic, functional, or radiographic characteristics.
| - | D + | DST | D + | TLIF | (D+DST) | - | (D+TLIF1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Age (yrs) | 140 | 65.8 | 7.7 | 66.1 | 47.4 | 80.0 | 147 | 64.1 | 9.0 | 64.7 | 31.5 | 80.7 | 0.08 | -2.05 | 2.20 |
| Height (cm) | 140 | 169.1 | 10.2 | 167.6 | 147.3 | 198.1 | 147 | 166.0 | 9.6 | 164.6 | 133.4 | 193.0 | 0.12 | -2.28 | 2.52 |
| Weight (kg) | 140 | 80.7 | 17.1 | 77.8 | 46.3 | 133.8 | 147 | 82.8 | 17.8 | 81.2 | 42.6 | 133.8 | 0.52 | -3.96 | 5.00 |
| BMI (k/m2) | 140 | 28.1 | 4.7 | 27.2 | 17.4 | 39.1 | 147 | 30.0 | 5.5 | 30.0 | 18.3 | 43.5 | 0.13 | -1.10 | 1.35 |
| CCI2 (w/ age points) | 140 | 2.5 | 1.3 | 2.0 | 0.0 | 7.0 | 147 | 2.4 | 1.3 | 2.0 | 0.0 | 8.0 | 0.05 | -0.29 | 0.40 |
| OST3 | 140 | 2.94 | 3.99 | 2.50 | -5.9 | 13.6 | 147 | 4.14 | 5.01 | 3.98 | -7.5 | 37.0 | -0.51 | -1.68 | 0.66 |
| Baseline Functional Status | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| ODI4 | 140 | 52.6 | 11.9 | 51.5 | 22.0 | 84.0 | 147 | 52.5 | 14.0 | 48.0 | 32.0 | 92.0 | 0.05 | -3.33 | 3.44 |
| VAS5 Back | 140 | 67.4 | 23.9 | 73.5 | 0.0 | 99.0 | 146 | 69.7 | 22.8 | 74.0 | 0.0 | 100.0 | 0.06 | -5.96 | 6.09 |
| VAS Worst Leg/Hip Pain | 140 | 78.8 | 13.2 | 81.0 | 22.0 | 100.0 | 134 | 79.9 | 15.4 | 82.5 | 31.0 | 100.0 | 0.02 | -3.71 | 3.76 |
| ZCQ Symptom Severity | 139 | 3.54 | 0.53 | 3.57 | 2.0 | 4.9 | 105 | 3.50 | 0.57 | 3.43 | 2.1 | 5.0 | 0.05 | -0.10 | 0.21 |
| ZCQ Physical Function | 139 | 2.75 | 0.44 | 2.80 | 1.4 | 3.6 | 105 | 2.80 | 0.52 | 2.80 | 1.0 | 4.0 | -0.05 | -0.19 | 0.08 |
| Gender | N | % | - | - | - | - | N | % | - | - | - | - | Diff. | LCL | UCL |
| Males | 59 | 42.1 | - | - | - | - | 44 | 29.9 | - | - | - | - | 0.1% | -12.4% | 12.5% |
| Females | 81 | 57.9 | - | - | - | - | 103 | 70.1 | - | - | - | - | . | . | . |
| Ethnicity | n | % | - | - | - | - | n | % | - | - | - | - | - | - | - |
| Hispanic or Latino | 13 | 9.3 | - | - | - | - | 10 | 6.8 | - | - | - | - | 0.4 | -6.1 | 6.9 |
| Not Hispanic or Latino | 127 | 90.7 | - | - | - | - | 137 | 93.2 | - | - | - | - | . | . | . |
| Race | n | % | - | - | - | - | n | % | - | - | - | - | p6 | - | - |
| White | 121 | 86.4 | - | - | - | - | 129 | 87.8 | - | - | - | - | <0.001 | - | - |
| Asian | 10 | 7.1 | - | - | - | - | 4 | 2.7 | - | - | - | - | - | - | - |
| Black | 2 | 1.4 | - | - | - | - | 14 | 9.5 | - | - | - | - | - | - | - |
| Native American | 0 | 0.0 | - | - | - | - | 0 | 0.0 | - | - | - | - | - | - | - |
| Pacific Islander | 0 | 0.0 | - | - | - | - | 0 | 0.0 | - | - | - | - | - | - | - |
| Other | 7 | 5.0 | - | - | - | - | 0 | 0.0 | - | - | - | - | - | - | - |
| Radiographic Assessments | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff | LCL | UCL |
| Angular Motion (deg) | 137 | 5.75 | 4.55 | 4.70 | 0.1 | 18.6 | 124 | 5.46 | 3.79 | 4.70 | 0.1 | 15.1 | 0.02 | -1.13 | 1.17 |
| Anterolisthesis (mm) | 140 | -4.63 | 2.60 | -4.7 | -10.6 | 2.6 | 142 | -4.97 | 2.61 | -5.1 | -12.3 | 1.7 | 0.10 | -0.57 | 0.78 |
| Translation (mm)7 | 137 | 1.30 | 1.07 | 1.10 | 0.0 | 4.9 | 124 | 1.26 | 0.97 | 1.00 | 0.0 | 5.1 | 0.02 | -0.26 | 0.30 |
| Disc Angle (deg)8 | 140 | 7.96 | 4.64 | 8.35 | -4.1 | 19.9 | 142 | 7.85 | 4.96 | 8.35 | -5.3 | 19.2 | -0.12 | -1.38 | 1.15 |
| Disc height (mm)9 | 140 | 6.87 | 1.91 | 7.00 | 1.4 | 11.3 | 142 | 7.02 | 2.06 | 7.05 | 1.2 | 11.7 | -0.02 | -0.54 | 0.50 |
| Facet Angle (deg)10 | 137 | 56.24 | 10.23 | 55.80 | 34.3 | 79.9 | 142 | 54.52 | 10.31 | 53.85 | 23.3 | 79.5 | 0.06 | -2.65 | 2.77 |
1 Treatment group differences and 95% confidence intervals (CI) for group differences, adjusted for PS subclass. Significant differences highlighted in gray.
2 Charleson Comorbidity Index [26]
3 Osteoporosis self-assessment tool
4 Oswestry Disability Index
5 Visual Analog Scale
6 p-value for chi-square test
7 Displacement of the posterior-inferior corner of the superior vertebra in a direction parallel to the superior endplate of the inferior vertebra, measured from flexion to extension on standing lateral radiographs.
8 The angle formed between the endplates of adjacent vertebrae, measured on neutral lateral radiographs to assess local segmental lordosis (lordosis>0°).
9 Simple mean of anterior and posterior disc heights, measured in neutral lateral x-rays.
10 Average angle of the facet joint articular surface from the coronal plane in axial lumbar CT or MRI slices.
| - | D + DST | D + TLIF | (D+DST) | - | (D+TLIF1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perioperative Outcomes | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Surgery time (min) | 140 | 112.0 | 32.1 | 105.5 | 59 | 216 | 142 | 187.3 | 75.9 | 175.0 | 56 | 476 | -70.1 | -85.3 | -54.9 |
| DST implant time (min) | 140 | 23.3 | 14.7 | 18.0 | 7 | 84 | - | - | - | - | - | - | - | - | - |
| Estimated Blood Loss (ml) | 140 | 52.2 | 53.3 | 45.0 | 5 | 350 | 146 | 240.4 | 231.4 | 200.0 | 0 | 1800 | -183.0 | -227.1 | -138.9 |
| Facility stay (nights) | 140 | 0.64 | 1.45 | 0.0 | 0 | 10 | 143 | 2.94 | 1.62 | 3.00 | 1 | 8 | -2.3 | -2.7 | -1.9 |
| Index Level of DS | n | % | - | - | - | - | n | % | - | - | - | - | p2 | – | - |
| L1-L2 | 0 | 0.0% | - | - | - | - | 0 | 0.0% | - | - | - | - | 0.004 | - | - |
| L2-L3 | 1 | 0.7% | - | - | - | - | 1 | 0.7% | - | - | - | - | - | - | - |
| L3-L4 | 20 | 14.3% | - | - | - | - | 8 | 5.4% | - | - | - | - | - | - | - |
| L4-L5 | 119 | 85.0% | - | - | - | - | 130 | 88.4% | - | - | - | - | - | - | - |
| L5-S1 | 0 | 0.0% | - | - | - | - | 8 | 5.4% | - | - | - | - | - | - | - |
1 Treatment group differences and 95% confidence intervals (CI) for group differences adjusted for PS subclass. Significant differences highlighted in gray.
2 p-value for chi-square test
3.2. Perioperative Outcomes
The index levels of degenerative spondylolisthesis and perioperative outcomes are summarized in Table 2. The index level of DS was predominantly L4/L5 in both groups: 85.0% of D + DST and 88.4% of D + TLIF. The D + DST group had a higher proportion of L3/L4 (14.3% vs 5.4%), whereas the D + TLIF group had more L5/S1 index levels vs. none in the D + DST group (5.4% vs. 0%), resulting in an overall significant difference in chi-square test between the groups.
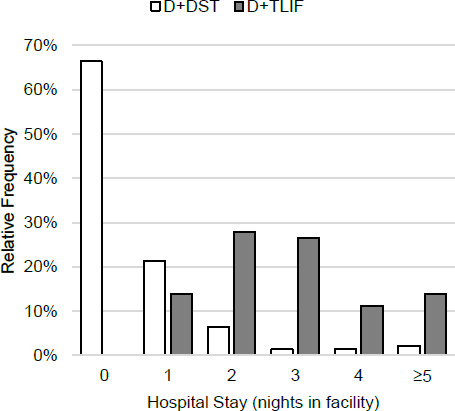
The D + DST had shorter surgery times by a PS-adjusted 70.1 minutes, lower EBL by 183 ml and shorter facility stay by 2.3 nights. The distribution of facility stay is shown in (Fig. 3). All differences were statistically significant. Median DST implantation took 18 minutes in addition to the decompression. In the D + DST group, 93/140 (66%) of patients were discharged the day of surgery vs. none of the D + TLIF patients, and 123/140 (88%) D + DST vs. 20/134 (14%) of D + TLIF patients were discharged by the first postoperative day. Fifty-seven (41%) of D + DST patients had their procedure in a surgery center setting. All patients treated in surgery centers were discharged directly without hospital admission.
3.3. Work Status and Return to Activities of Daily Living (ADL)
Work and ADL status are summarized in Table 3. Preoperatively, there were no differences between groups in the proportion of patients working or not working due to their spinal condition. At both the sixth week and third month postoperatively, a significantly greater proportion of the D + DST patients were working. Of the patients not working, a significantly greater proportion of the D + TLIF patients were not working due to their spinal condition at both the 6-week and 3-month follow-up visits. Of patients who were working preoperatively, 90% of D + DST and 47% of D + TLIF patients had returned to work by day 90.
At each visit, patients were asked whether they had returned to their normal ADLs since their previous visit, and if so, how many weeks post-surgery they returned to ADLs. In both intervals (from discharge to 6 weeks and 6 weeks to 3 months), a significantly greater proportion of D + DST patients reported that they had returned to their normal ADLs. At 90 days postoperatively, 89% of D + DST and 57% of D + TLIF patients had returned to normal ADLs.
3.4. Patient-reported Clinical Outcomes
Patient-reported outcomes through 3-months follow-up are presented in Table 4. Both groups demonstrated significant improvements in disability, back and leg pain within the first three months. At week 6, the D + DST group had significantly lower ODI scores and a significantly greater proportion of the D + DST group patients had achieved a 15-point improvement in ODI from their preoperative condition.
3.5. Safety
Safety outcomes are summarized in Table 5. Two D + DST (1.4%) and four D + TLIF (2.7%) patients did not have the index procedure successfully completed. Both D + DST patients had intraoperative spinous process fractures that prevented DST placement. The procedures were completed as laminectomies, with one patient electing to proceed to a posterolateral fusion on the first postoperative day. In the D + TLIF group, the interbody cage could not be placed in three patients due to neurologic injury (n=2) or other intraoperative considerations (1), while one patient experienced an intraoperative myocardial infarction that resulted in the procedure being aborted.
Two D + DST patients (1.5%) and three D + TLIF patients (2.1%) had reoperations within the first 90 days. Of the D + DST patients, one had debridement and revision decompression due to a staph infection, and one had additional decompression on the contralateral side to the index decompression due to progressive symptoms, leaving the DST in place. Of the D + TLIF patients, one was revised to L3-S1 posterior fusion after pedicle screw and cage migration causing radicular pain; one had wound complications requiring inpatient irrigation; and one had a postoperative CSF leak that required return to the OR, exploration and dural repair.
There were no significant differences in the rates of intraoperative dural tear, surgical wound infection or wound complications. Through three months follow-up, there were 29 SAEs in the D + DST group and 28 in the D + TLIF group.
| - | D + DST (N=140) | D + TLIF (N=147) | (D+DST) | - | (D+TLIF1) | ||
|---|---|---|---|---|---|---|---|
| Patients working | n | % | n | % | Diff. | LCL | UCL |
| Pre-Op | 70 | 50.0% | 59 | 41.5% | 0.2% | -12.4% | 12.7% |
| Week 6 | 46 | 34.3% | 18 | 14.1% | 20.3% | 10.2% | 30.3% |
| Month 3 | 62 | 47.3% | 36 | 28.8% | 18.5% | 6.9% | 30.2% |
| If not working, due to patient’s spinal condition? | n | % | n | % | p2 | - | - |
| Pre-Op | 10 | 14.3% | 15 | 18.1% | 0.525 | - | - |
| Week 6 | 22 | 25.3% | 48 | 43.6% | 0.016 | - | - |
| Month 3 | 8 | 11.6% | 28 | 31.5% | 0.003 | - | - |
| Patients returned to ADL | n | % | n | % | p2 | - | - |
| Discharge to Week 6 | 87 | 64.4% | 40 | 30.1% | <0.001 | - | - |
| Week 6 to Month 3 | 35 | 26.7% | 35 | 26.9% | <0.001 | - | - |
1 Treatment group differences and 95% confidence intervals (CI) for group differences adjusted for PS subclass. Significant differences are highlighted in gray.
2 p-value for chi-square test
| - | D + DST | D + TLIF | (D+DST) | - | (D+TLIF1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ODI | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Week 6 | 132 | -30.9 | 18.2 | -30.5 | -68.0 | 18.0 | 123 | -18.5 | 19.3 | -20.0 | -70.0 | 33.0 | -10.7 | -15.9 | -5.5 |
| Month 3 | 128 | -36.2 | 17.5 | -38.0 | -80.0 | 10.0 | 119 | -30.3 | 19.1 | -30.0 | -92.0 | 11.0 | -4.2 | -9.2 | 0.8 |
| ODI Success2 | N | n | % | - | - | - | N | n | % | - | - | - | Diff. | LCL | UCL |
| Week 6 | 132 | 106 | 80.3% | - | - | - | 123 | 74 | 60.2% | - | - | - | 15.7 | 3.9 | 27.5 |
| Month 3 | 128 | 116 | 90.6% | - | - | - | 119 | 92 | 77.3% | - | - | - | 9.0 | -0.5 | 18.5 |
| VAS Back Pain Improvement | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Week 6 | 132 | -46.1 | 28.1 | -52.0 | -93 | 45 | 121 | -41.6 | 30.0 | -42.0 | -99 | 34 | -3.2 | -11.3 | 4.8 |
| Month 3 | 128 | -47.3 | 29.6 | -55.0 | -93 | 52 | 116 | -45.6 | 30.5 | -48.5 | -100 | 32 | -1.7 | -10.0 | 6.7 |
| VAS leg/hip pain improvment3 | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Week 6 | 132 | -55.9 | 25.0 | -63.0 | -100 | 7 | 112 | -53.6 | 33.2 | -60.5 | -100 | 17 | -1.2 | -9.3 | 6.8 |
| Month 3 | 128 | -52.2 | 31.5 | -62.5 | -100 | 51 | 108 | -57.0 | 32.2 | -67.0 | -100 | 32 | 5.9 | -3.0 | 14.8 |
| ZCQ Symptom Severity | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Week 6 | 131 | -1.49 | 0.74 | -1.57 | -3.6 | 0.1 | 94 | -1.28 | 0.82 | -1.29 | -3.9 | 0.6 | -0.16 | -0.39 | 0.07 |
| Month 3 | 127 | -1.53 | 0.76 | -1.57 | -3.3 | 0.9 | 92 | -1.55 | 0.80 | -1.50 | -3.4 | 0.6 | 0.03 | -0.20 | 0.26 |
| ZCQ Physical Function | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Week 6 | 131 | -1.09 | 0.61 | -1.20 | -2.2 | 1.0 | 94 | -0.88 | 0.73 | -1.00 | -2.8 | 1.4 | -0.15 | -0.35 | 0.04 |
| Month 3 | 127 | -1.21 | 0.63 | -1.40 | -2.4 | 0.2 | 92 | -1.22 | 0.67 | -1.20 | -2.8 | 0.4 | 0.09 | -0.10 | 0.28 |
| ZCQ Satisfaction Scores | N | Mean | SD | Med | Min | Max | N | Mean | SD | Med | Min | Max | Diff. | LCL | UCL |
| Week 6 | 132 | 1.53 | 0.55 | 1.33 | 1.00 | 3.17 | 97 | 1.51 | 0.55 | 1.33 | 1.00 | 4.00 | 0.04 | -0.12 | 0.21 |
| Month 3 | 128 | 1.52 | 0.58 | 1.33 | 1.00 | 3.50 | 95 | 1.48 | 0.54 | 1.33 | 1.00 | 4.00 | 0.07 | -0.10 | 0.24 |
1 Treatment group differences and 95% confidence intervals (CI) for group differences adjusted for PS subclass. Significant differences highlighted in gray.
2 Patients achieving a 15-point improvement in ODI from preoperative.
3 Worst side reported.
| - | D + DST | D + TLIF | (D+DST) | - | (D+TLIF) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Index procedure success & 90-day reoperations | N | n | % | N | n | % | Diff. | LCL | UCL |
| Successful index procedure | 140 | 138 | 98.6% | 147 | 143 | 97.3% | 1.1% | -1.8% | 4.0% |
| Reoperations within 90 days | 138 | 2 | 1.5% | 143 | 3 | 2.1% | - | - | - |
| Initial Surgical complications | N | n | % | N | n | % | Diff. | LCL | UCL |
| Dural tear | 140 | 6 | 4.3% | 10 | 10 | 6.8% | -2.5% | -7.8% | 2.8% |
| Surgical wound infection | 140 | 1 | 0.7% | 4 | 4 | 2.7% | -2.0% | -5.0% | 1.0% |
| Wound complications dehiscence, bruising-and soft tissue damage | 140 | 6 | 4.3% | 6 | 6 | 4.1% | 0.2% | -4.4% | 4.8% |
| Discharge to wk 6 | Week 6 to Mo 3 | - | - | - | - | - | |||
| Specific Serious Adverse Events | D + DST | D + TLIF | D + DST | D + TLIF | - | - | - | - | - |
| Lumbar spine-related SAEs | - | - | - | - | - | - | - | - | - |
| Leg weakness or numbness | 1 | - | - | - | - | - | - | - | - |
| CSF leak | 1 | 1 | - | 1 | - | - | - | - | - |
| Surgical wound infection | 1 | 1 | - | - | - | - | - | ||
| Dural tear | 1 | - | - | - | - | - | - | - | - |
| Lumbar spinal stenosis lumbar requiring additional decompression | 1 | - | 1 | - | - | - | - | - | - |
| New/increased leg pain | - | 1 | - | - | - | - | - | - | - |
| Spinous process fracture affecting device fixation | 1 | - | - | - | - | - | - | - | - |
| Subsidence or displacement of interbody cage | - | 1 | - | - | - | - | - | - | - |
| Radiculopathy | 4 | 1 | 2 | - | - | - | - | - | - |
| Non lumbar spine-related SAEs | - | - | - | - | - | - | - | - | - |
| Pneumonia | - | 1 | - | - | - | - | - | - | - |
| Pulmonary Embolism | 1 | 3 | - | - | - | - | - | - | - |
| Arrhythmia | - | 3 | - | - | - | - | - | - | - |
| Cardiogenic shock | 1 | - | - | - | - | - | - | - | - |
| Congestive Heart Failure | - | - | - | 1 | - | - | - | - | - |
| Deep Vein Thrombosis | - | 1 | - | - | - | - | - | - | - |
| Osteoarthritis, Hip | - | 2 | - | - | - | - | - | - | - |
| Osteoarthritis, Knee | 1 | 2 | 1 | - | - | - | - | - | |
| Osteoarthritis, other | - | - | 1 | - | - | - | - | - | - |
| Fever | - | 1 | - | - | - | - | - | - | - |
| Ileus or intestinal obstruction | - | 1 | - | - | - | - | - | - | - |
| Anemia | - | 2 | - | - | - | - | - | - | - |
| Other respiratory disorder | - | 1 | - | - | - | - | - | - | - |
| Encephalopathy, acute | 1 | 1 | - | - | - | - | - | - | - |
| Other nervous system disorder | - | 1 | - | - | - | - | - | - | - |
| Disorders of nose; includes pain | - | - | - | 1 | - | - | - | - | - |
| Hernia | - | 1 | - | - | - | - | - | - | - |
| Disorder of intestines | 1 | - | - | - | - | - | - | - | - |
| Renal failure | 1 | - | - | - | - | - | - | - | - |
| Hematuria | 1 | - | - | - | - | - | - | - | - |
| Urinary Tract Infection | 1 | - | - | - | - | - | - | - | - |
| Urinary retention | 1 | - | - | - | - | - | - | - | - |
| Trauma | 1 | - | 1 | - | - | - | - | - | - |
| Cancer | 1 | - | - | - | - | - | - | ||
| Thoracic Stenosis | 1 | - | - | - | - | - | - | ||
| Cervical Stenosis | - | - | 1 | - | - | - | - | - | - |
| Total | 22 | 25 | 7 | 3 | - | - | - | - | - |
4. DISCUSSION
These perioperative outcomes demonstrate that patients receiving D + DST for symptomatic DS with a range of instability had faster, less invasive procedures compared to similar patients receiving D + TLIF due to the shorter procedure time, more limited dissection and lower blood loss. With the majority of D + DST patients discharged the same day and 88% discharged by the first postoperative day, D + DST appears well suited for the outpatient setting. A significant portion of the D + DST group had their procedures in a surgery center. A recent shift in spine procedures to ambulatory surgery center (ASC) settings was accelerated by the COVID-19 pandemic and is likely to persist due to patients’ preference for the smaller institutional setting and more personalized care, physicians’ preference for operational flexibility and control, and the potential cost savings to payors. A primary concern when moving procedures to the ASC setting is safety. All 57 D + DST patients treated in surgery centers were discharged directly, without hospital admission, indicating that the D + DST procedure is appropriate for the ASC setting in properly selected patients.
Compared to D + TLIF, the D + DST group demonstrated faster recovery. While both groups achieved significant improvement in leg/hip and back pain as well as function by month three, the D + DST group had a significantly lower disability and a greater proportion had achieved a 15-point improvement in disability at six weeks. Similarly, more D + DST patients reported returning to work and ADLs by the 6-week and 3-month intervals and fewer D + DST patients were not working due to their spinal conditions. Moreover, the Kaplan-Meier analyses demonstrated faster overall return to work and ADLs for the D + DST group over the 90-day postoperative period. We hypothesize that both groups experience early relief of neurologic symptoms due to direct decompression, resulting in comparable, significant improvements in VAS pain. The faster ODI improvement in the investigational group is likely due to the less invasive nature of the DST procedure and, conversely the incisional pain, activity and postural limitations during recovery from the fusion procedure. This is consistent with the faster return to work and ADLs reported by the D + DST group.
The safety profiles of both groups were very similar. Some risks and adverse events are specific to the treatment group, such as intraoperative spinous process fracture in the D + DST group and screw/cage migration in the D + TLIF group, while others, such as infection or recurrent symptoms, are risks of any spinal surgery. The relatively low incidence of serious adverse events in either group indicates that the D + DST surgery does not pose unacceptable surgical risks.
It is important to recognize that these outcomes represent only early results, as the intent of this report is to assess the relative invasiveness, safety and short-term clinical outcomes indicative of basic procedural success and surgical recovery. The relative merits of each procedure for overall, long-term clinical effectiveness will be evaluated per the established IDE study protocol.
When the neurologic symptoms from DS can be resolved by direct surgical decompression, the addition of a stabilization system that addresses the underlying instability without creating new problems, including loss of motion and flexibility, would be ideal. Peul et al. described the limitations and disadvantages of fusion and that it may be over-utilized in many cases, yet acknowledge that fusion is appropriate in the presence of instability 27]. Fusion is invasive, requires an extended hospital stay, and entails a lengthy recovery period – all driving up the cost of care, while the shift to value-based healthcare is accelerating pressure to improve clinical outcomes, patient satisfaction and lower the cost of care [28]. With the aging population, a growing number of patients will experience degenerative conditions such as DS and will seek out surgery to address the resulting pain, increasing the need for treatment options that meet value-based constraints. The DST may provide an alternative to fusion for this patient population that meets the needs of patients, physicians, payors and society for a procedure with faster recovery, durable stabilization and lower costs.
CONCLUSION
In the treatment of symptomatic DS, durable relief of neurologic symptoms is the primary goal, accomplished by neural decompression. The purpose of adding fusion to stabilize the segment is to maintain the effectiveness of the decompression; it has also been proposed as possibly effective in the effort to relieve mechanical pain. The objective of the DST is to maintain normal or near-normal motion in an attempt to reduce adverse outcomes commonly seen with conventional spinal fusion, most notably the development of adjacent-segment disease. The rationale behind this approach is similar to that used in hip and knee arthroplasty. The functional spinal unit is more complicated, however; the replication of normal spinal motion has been challenging. The DST was designed to restore natural motion and stability after decompression by augmenting the posterior tension band, creating an elastic resistance to flexion and maintaining lordosis.
These results demonstrate that the DST procedure is faster, less invasive and more amenable to the outpatient setting compared to an interbody fusion. While both the D + DST and D + TLIF groups demonstrated significant clinical improvement in 3 months, the D + DST group recovered faster, with a faster return to work and ADLs. While these initial results do not establish long-term effectiveness yet, they demonstrate the advantages and value of this procedure if the clinical outcomes are durable. The long-term comparative effectiveness of the D + DST vs. D + TLIF procedures must be confirmed through the complete IDE study protocol, however, these results indicate the potential of this procedure and technology.
LIST OF ABBREVIATIONS
| ADL | = Activities of daily living |
| ASC | = Ambulatory surgery center |
| BMI | = Body mass index |
| CCI | = Charleson comorbidity index |
| CI | = Confidence interval |
| CSF | = Cerebrospinal fluid |
| DS | = Degenerative spondylolisthesis |
| DST | = Dynamic sagittal tether |
| D + DST | = Decompression and dynamic sagittal tether stabilization |
| D + TLIF | = Decompression and transforaminal lumbar interbody fusion |
| EBL | = Estimated blood loss |
| FDA | = United States Food and Drug Administration |
| IDE | = Investigational device exemption |
| ODI | = Oswestry disability index |
| OST | = An osteoporosis screening tool |
| PS | = A propensity score |
| SAE | = Serious adverse event |
| TLIF | = Transforaminal lumbar interbody fusion |
| VAS | = Visual analog scale |
| ZCQ | = Zurich claudication questionnaire |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Institutional Review Board (IRB) approval was obtained for each site, whether through the sites' local IRB or through the central WCG IRB. WCG IRB central approval corresponds to protocol # 20170381.
HUMAN AND ANIMAL RIGHTS
No animals were used for studies that are the basis of this research. All human procedures followed were in accordance with the Helsinki Declaration of 1975.
CONSENT FOR PUBLICATION
Informed consent was received from the participants.
STANDARDS FOR REPORTING
CONSORT guidelines and methodology were followed.
FUNDING
Empirical Spine, Inc. sponsored this work as part of a US Food and Drug Administration (FDA) investigational device exemption (IDE) study.
AVAILABILITY OF DATA AND MATERIALS
The data and supportive information are available within the article.
CONFLICT OF INTEREST
Investigators performing patient screening, enrollment/consent and assessment/collection of outcomes had no conflicts other than institutional research support. Authors TFA and LCF have equity interest, royalties and employment/consulting agreements with Empirical Spine. GM is a consultant to Empirical Spine.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the following individuals for their contributions to this work: Ravinder-Raj Bains, MD, Sigurd Berven, MD, Sigita Burneikiene, MD, Eugene Carragee, MD, Jens Chapman, MD, Susmita Chatterjee, Ivan Cheng, MD, Dennis G Crandall, MD, Reginald J Davis, MD, Harel Deutsch, MD, Jeffrey Fischgrund, MD, Jeffrey L Gum, MD, Richard G Guyer, MD, Bob Hachadoorian, Ph.D., Hamid Hassanzadeh, MD, Serena Hu, MD, M Agnes Ith, MD, Brendan Keenan, Ph.D., Calvin C Kuo, MD, Matt Lessin, Matthew Mermer, MD, Umesh Metkar, MD, Rod J Oskouian, MD, Mick Perez-Cruet, MD, MS, Sharad Rajpal, MD, W Zack Ray, MD, Harvinder Sandhu, MD, Sandra Schlachter, Ph.D., Khalid Sethi, MD, Adam L Shimer, MD, Michael P Stauff, MD, Richard Treadwell, Sheetal Vinayek, Michael Y Wang, MD, Andrew P White, MD, S Tim Yoon, MD, Ph.D., Elizabeth Yu, MD, Marcia Wachna, RN
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher's website along with the published article.


