All published articles of this journal are available on ScienceDirect.
Tissue Engineered Strategies for Pseudoarthrosis
Abstract
Numerous classification systems of non-union have been proposed based on: presence or absence of infection, radiographic features, clinical findings, biologic activity, location and shape. The management of pseudarthrosis is strongly related to the type of non-union (infected versus uninfected, atrophic versus hypertrophic). Surgical management of pseudarthrosis is generally effective with a success rate ranging from 75 to 100%. Nevertheless, in a relatively high number of instances several combined treatments are required for the fracture healing. The current gold standard to stimulate the bone regeneration is represented by the revision surgery with the application of autologous bone grafts. However, several approaches have been described to promote and enhance the bone tissue regeneration, including extracorporeal shock wave therapy (ESWT), ultrasound, electromagnetic, bone morphogenic proteins (BMPs) and platelet-rich-plasma (PRP).
The aim of the present study was to perform a systematic review of the literature evaluating the current therapies to promote and enhance the bone tissue healing. The systematic review was performed according to PRISMA guidelines with a PRISMA checklist and algorithm.
Limitations of the present systematic review are mainly related to the scanty quality of the studies available in the literature. Although the therapies previously described for the management of patients with non-unions seems to be effective, the limitations of the included studies, especially the extensive clinical heterogeneity, make not possible to provide clear recommendations regarding the application of these approaches. The problems remain the need to better understand the most effective treatment options, subject to surgical stabilization as a first step.
INTRODUCTION
The term of pseudarthrosis or non-union is usually applied to fractures which do not consolidate within a period between 6 and 8 months [1]. On the other hand, the term of delayed union indicates fractures which consolidate in a period longer than normal [2] Both conditions are characterized by specific clinical and radiological signs.
Numerous classification systems of non-union have been proposed based on: presence or absence of infection, radiographic features, clinical findings, biologic activity, location and shape. The AO classification includes: hypertrophic non-union, consisting of a false joint where a fibrocartilaginous cavity is lined with synovium producing synovial fluid; avascular/avital non-union with or without bone loss, resulting from injury and/or surgery which lead to devascularization of the bone fragments; atrophic non-union, which is a vascularized non-union secondary to marked instability resulting in resorption of bone fragment and rounding of their ends.
The management of pseudarthrosis is strongly related to the type of non-union (infected versus uninfected, atrophic versus hypertrophic) [3, 4]. The intramedullary nailing is usually preferred for an uninfected non-union of long bone shaft. On the other hand, the external fixation is indicated in patients with an infected non-union of long bone shaft. In patients with closed non-unions, the management with compression plates has been also investigated [5-8]. In case of hypertrophic non-unions, high success rates have been reported with compression plates alone, whereas supplementary bone-grafting seems to be required for atrophic non-unions. Finally, the Ilizarov technique has been used for the management of angulated malunions and failures of union associated with malalignment.
Surgical management of pseudarthrosis is generally effective with a success rate ranging from 75 to 100% [9-5]. Nevertheless, in a relatively high number of instances several combined treatments are required for the fracture healing. The current gold standard to stimulate the bone regeneration is represented by the revision surgery with the application of autologous bone grafts, usually from the iliac crest, with or without replacement of the fracture fixation 0 [16]. However, several approaches have been described to promote and enhance the bone tissue regeneration, including extracorporeal shock wave therapy (ESWT), ultrasound, electromagnetic, bone morphogenic proteins (BMPs) and platelet-rich-plasma (PRP).
The aim of the present study was to perform a systematic review of the literature evaluating the current therapies to promote and enhance the bone tissue healing. The feasibility and the effectiveness of these were also assessed.
MATERIALS AND METHODS
Electronic Literature Search
The systematic review was performed according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses) guidelines with a PRISMA checklist and algorithm [17, 18], and already validated in our setting [19-36]. Three independent reviewers (UGL, UT, and ML) separately conducted the search. All journals were considered, and all relevant articles were analyzed. Only articles published in a peer-reviewed journal were included. All articles were initially screened for relevance by title and abstract, excluding articles without an abstract, and obtaining full-text article if the abstract did not allow the authors to assess the defined inclusion and exclusion criteria. The three authors (UGL, UT, and ML) reviewed the abstract of each publication, than performed a close reading of all papers and extracted data, to minimize selection bias and errors. A cross reference research of the selected articles was also performed to obtain other relevant articles for the study.
The search was performed on 1st April 2012. The following databases were screened: Medline, Google Scholar, EMBASE and Ovid. All articles reporting outcomes on tissue engineered strategies, performed singularly or in combination with other surgical procedures, for the management of non-unions have been included. Given the linguistic capabilities of the authors, articles in English, French, Spanish, German or Italian were included. According to the Oxford centre of EBM, level I, II, III, IV articles were found in the literature and included in our study. Literature reviews, case reports, studies on animals, cadavers or in vitro, biomechanical reports, tumoral studies, technical notes, letters to editors and instructional course were excluded.
Finally, to avoid bias, the selected articles, the relative list of references and the articles excluded from the study were reviewed, assessed and discussed by all the authors and if there was disagreement among authors regarding inclusion and exclusion criteria the senior authors (NM and VD) made the final decision.
The inclusion and exclusion criteria for study analysis, the check-list and the search algorithm according to PRISMA guidelines are respectively given in Tables 1 and 2 and Fig. (1).
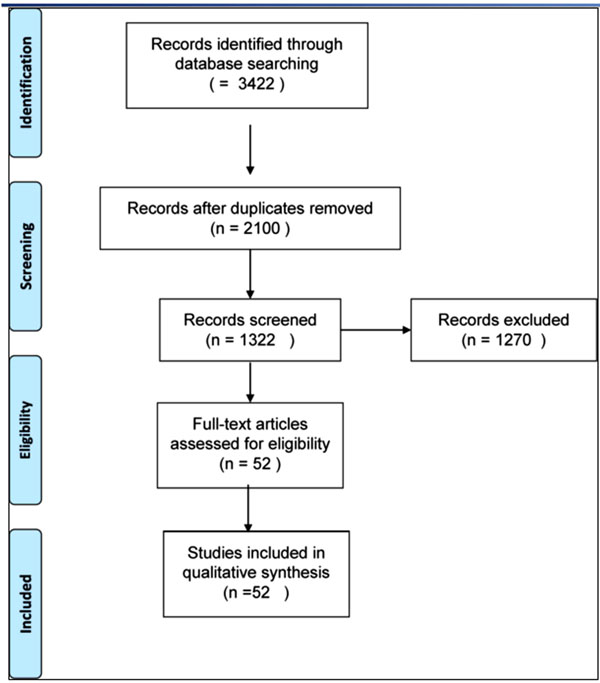
PRISMA Flow diagram of the literature search.
PRISMA Check List
| Section/Topic | # | Checklist Item | Reported on Page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design. | |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | |
Inclusion and Exclusion Criteria
| INCLUSION CRITERIA | |
| DATABASES | Medline, Google Scholar, EMBASE, Ovid |
| SOURCH DATE/PUBDATE | 1st April 2012/1982-2011 |
| ARTICLE’S LENGUAGE | English, French, Spanish, German, Italian |
| LEVEL OF EVIDENCE | Oxford centre of EBM, level I, II, III, IV |
| DIAGNOSIS | Non-unions results of fractures |
| LESION ASSESSMENT | Imaging: MRI, TC Surgical: Open |
| TYPE OF SURGERY | stabilization of the fracture, debridement and application of tissue engineered |
| OUTCOMES ASSESSMENT | Clinical: Clinical examination Imaging: MRI, TC evaluation |
| MINIMUM FOLLOW-UP TIME | 6 months |
| EXCLUSION CRITERIA | Literature reviews, case reports, studies on animals, cadavers or in vitro, biomechanical reports, tumoral studies, technical notes, letters to editors and instructional course |
| TYPE OF STUDY | All articles reporting outcomes on tissue engineered strategies, performed singularly or in combination with other surgical procedures, for the management of non-unions. |
| OUTCOMES MESURES | no information on diagnosis, follow up, imaging of the non-unions, clinical examination, clinical post operative outcomes, statistical analysis of the relative outcomes. |
RESULTS
The literature search and cross-referencing resulted in a total of 3422 references of which 2100 were rejected due to off topic abstract and/or failure to respect inclusion criteria (Fig. 1).
After reading the remaining full text articles, another 1270 were excluded because of insufficient details and uncertain diagnosis and outcomes measures. The remaining 52 articles, describing a total of 1411 non-union in 1410 patients, were included in the study.
Extracorporeal Shock Wave Therapy
Three of the included studies investigated ESWT [37-39], including 325 patients (230 males and 95 females) with an average age of 41.7 years at the time of treatment. Patients were evaluated at an average follow-up of 12 months. ESWT was effective in 249 of 325 (77%) patients. On the other hands, there was a failure in 59 (18%) patients and unknown result in 17 (5%). In the included studies, no major adverse side effects were recorded in association with the ESWT or the subsequent period of immobilization.
Ultrasound
Four studies evaluating the ultrasound therapy were taken into account [40-43]. The total number of patients undergoing ultrasound was 104 (71 males and 33 females) with an average age of 38.48 years at the time of treatment. This approach resulted effective in 70 (67%) patients. There was a failure in 3 (3%) patients and unknown result in 17 (16%). No treatment-related complications were reported in these studies.
Electromagnetic
Ten studies investigating electromagnetic were taken into account, including 329 patients [44-53] (172 males, 67 females and 90 with unknown gender) with an average age of 36.93 years at the time of treatment, ranging from 32 to 46 years. Patients were assessed at an average follow-up of 9.8 months (ranged from 12 weeks to 120 months). In five studies, including 178 patients, the management consisted in a combination of surgery and electromagnetic. This approach resulted successful in 134 (77%) patients and uneffective in 44 (23%). In the remaining five studies, a comparison between electromagnetic alone and electromagnetic associated with surgery has been performed. The association of these treatments resulted in better results than electromagnetic approach alone (success rate 75% - 57 of 76 patients - versus 43% - 32 of 75 patients - and failure rate 25% - 19 of 76 patients - versus 57% - 43 of 75 patients).
Bone Morphogenic Proteins
Ten studies investigated BMPs [10, 54-61], including 630 patients (369 males, 178 females, and 83 with unknown gender) with an average age of 50.06 years at the time of treatment, ranging from 35.3 to 56.6 years. Patients were assessed at an average follow-up of 18.45 months (ranged from 12 to 29.2 months).
In five studies assessing the use of BMPs alone for the management of nonunion, the success rate was 88% - 259 of 294 patients - and failure rate was 12% - 35 of 294 patients. Three studies performed a comparison between BMPs alone and BMPs associated with autologus bone graft. The BMPs alone resulted in better results than the association of these treatments (success rate 87% - 71 of 82 patients - versus 58% - 63 of 108 patients - and failure rate 13% - 11 of 82 patients - versus 10% - 11 of 108 patients). Two studies performed a comparison between BMPs alone and BMPs associated with PRP. The BMPs alone resulted in better results than the association of these treatments (success rate 96% - 73 of 76 patients - versus 67% - 49 of 73 patients - and failure rate 12% - 9 of 76 patients - versus 33% - 24 of 73 patients).
Platelet-Rich-Plasma
Three studies evaluated the use of PRP for the management of non-union [56, 57, 62], including 95 patients (38 males, 34 females and 23 with unknown gender) with an average age of 39 years at the time of treatment. Patients were assessed at an average follow-up of 13 months. One study investigated the use of PRP alone in 22 patients, reporting a success rate of 91% (20 of 22 patients) and a failure rate of 9% (2 of 22 patients). On the other hand, two studies showed that the use of PRP with BMPs provide worse outcomes than BMPs alone.
DISCUSSION
The current gold standard management of non-unions consists of revision surgery with the application of autologus bone grafts with or without replacement of the fracture fixation. However, in the last decades, several approaches have been described to promote the bone tissue regeneration, including extracorporeal shock wave therapy (ESWT), ultrasound, electromagnetic, bone morphogenic proteins (BMPs) and platelet-rich-plasma (PRP).
The BMPs are members of the transforming growth factor-beta (TGF-b) superfamily with a great osteoinductive potential. They induce a sequential cascade of events for chondro-osteogenesis during the bone formation and fracture healing process, including chemotaxis [63], proliferation of mesenchymal and osteoprogenitor cells [1], and their differentiation into a chondrogenic or osteogenic lineage [64, 65]. Currently, two of the 16 different BMP-homologous human molecules28 have been used in several clinical trials and are commercially available [66, 67]. RhBMP-7 or OP-1 received FDA approval for use in patients with recalcitrant long bone non-unions where autograft is unfeasible and alternative treatments have failed. The rhBMP-2 has been approved for the acute treatment of open tibial fractures associated with the intramedullary nail [68]. The results of the included studies confirm the efficacy of BMP-7 for the management of nonunion reporting a success rate around 88%. Moreover, the effectiveness of the BMPs alone is greater than those of a combination of BMPs and PRP or BMPs and autologus bone graft. The BMP application can be associated with side effects, such as local erythema and swelling, heterotopic ossification and immune reactions. The use of BMP-7 is contraindicated in children and patients who are pregnant or have autoimmune deficiencies, or patients underwent immunosuppressive therapies.
The PRP application is related to the important role played by platelets in fracture healing. Indeed, the alpha granules of platelets, releasing several growth factors in the fracture rim, such as platelet-derived growth factor (PDGF), transforming growth factor beta (TGF-b), fibroblast growth factor (FGF-b), and vascular endothelial growth factor (VEGF) stimulate polymorphonuclear leukocytes, lymphocytes, monocytes, and macrophages [69]. TGF-b and PDGF molecules show in vivo osteoinductive capacity [70, 71]. Moreover, VEGF enhances bone formation and bone healing by improving angiogenesis [72], and appears to be an appropriate tool to induce bone healing in atrophic non-unions [73]. Among the three included studies evaluating the effectiveness of PRP, two assessed the combination of PRP with BMPs compared with the use of BMPs alone [3, 57]. Their findings suggest that the rhBMP-7 as a bone-stimulating agent for the management of non-unions of the long bones is superior to the association of PRP and rhBMP-7 with regard to their clinical and radiological efficacy.
The electromagnetic system consists in an electric current applied at the site of the fracture. The electromagnetic coils are applied over the fracture and are connected to the portable generator. The tension of the coils is set before starting treatment. During the treatment the fractured bone is immobilized. According to the results of the included studies, electromagnetic associated with surgery provide better results than electromagnetic approach alone (success rate 75% versus 43%). Despite its good results, some authors do not recommend the use of electromagnetic stimulation as a first line treatment in unselected patients with delayed unions or non-unions, but suggest its application in patients that fail the conventional treatments.
ESWT can play a role in the management of non-union because they produce microfractures within the bone, stimulating neovascularization, osteoblast proliferation and activation, and synthesis of bone tissue. Delius et al. showed that shock waves produce radiographic lucencies in the bone marrow, intense formation of new cortical bone, and minor trabecular remodeling but did not cause gross fractures. ESWT is a safe and effective method for the treatment of delayed unions and pseudoarthrosis. In the included studies, the rate of success for the management of pseudoarthrosis was around 77%. This approach should be taken into account in every case of bone union disturbances as, under favourable conditions, it may help to avoid surgery. Surgery should be performed whenever there is excessive displacement, high instability of bony fragments, and in the presence of bone defects.
Low intensity pulsed ultrasound (LIPUS) is a type of mechanical energy transmitted transcutaneously by high-frequency acoustic pressure waves. The intensity of LIPUS is within the range of ultrasound intensities used for diagnostic purposes and is regarded as non-thermal and non-destructie. Bone cells are sensitive to strains caused by physical loading. Mechanoreceptors convert biophysical stimuli into biochemical responses that modify gene expression and cellular adaptation. The micro-mechanical stress produced by LIPUS may provide a surrogate for the forces normally applied on bone by physical loading according to Wolffs' law. LIPUS increases prostaglandin E2 synthesis by the induction of cyclooxygenase-2 in osteoblastic cells in vitro. Randomized clinical trials showed acceleration of clinical fracture healing by LIPUS in fresh fractures and osteotomies. LIPUS also restores the disrupted fracture healing process in non-union cases. The exact mechanism by which LIPUS affects clinical bone healing is however still unknown. The positive effect of LIPUS on fracture healing may be caused by a stimulation of the different cellular processes involved in fracture repair and bone formation, such as angiogenesis, chondrogenesis, and intramembranous and endochondral ossification.
Limitations of the present systematic review are mainly related to the scanty quality of the studies available in the literature. Moreover, as the data from the available studies were often poorly reported, it was not possible to perform a comprehensive pooling of data.
Although the therapies previously described for the management of patients with delayed and non-unions seems to be effective, the limitations of the included studies, especially the extensive clinical heterogeneity, make not possible to provide clear recommendations regarding the application of these approaches. The problems remain the need to better understand the most effective treatment options, subject to surgical stabilization as a first step. Clearly, studies of higher levels of evidence, including large randomised trials, should be conducted to help answer these questions. Future trials should use validated functional and clinical outcomes, adequate methodology, and be sufficiently powered.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


