RESEARCH ARTICLE
The Effect of Anterior Cruciate Ligament Reconstruction on the Progression of Osteoarthritis
Rory Norris 1, Pete Thompson 1, Alan Getgood*, 2
Article Information
Identifiers and Pagination:
Year: 2012Volume: 6
Issue: Suppl 3
First Page: 506
Last Page: 510
Publisher ID: TOORTHJ-6-506
DOI: 10.2174/1874325001206010506
Article History:
Received Date: 6/7/2012Revision Received Date: 9/9/2012
Acceptance Date: 17/9/2012
Electronic publication date: 30/11/2012
Collection year: 2012

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Anterior cruciate ligament rupture (ACL) is a common injury, particularly among young sporting adults. Early onset osteoarthritis (OA) can be a devastating and difficult to manage consequence of such an injury. The techniques for reconstructing the ACL are advancing all the time, but the effect that this has on the progression of OA is less well understood. Many factors affect the development of OA following an ACL injury, including direct and indirect trauma to the articular cartilage, associated meniscal injuries, chronic tibiofemoral joint instability, and multiple enzymatic pathways. This review will summarize the current evidence surrounding each of these areas, and describe some of the recent developments that may have an impact on the management of these injuries in the future.
INTRODUCTION
Anterior cruciate ligament (ACL) rupture is a common injury among young sportsmen and sportswomen, with a reported annual incidence in the general population of 0.8 per 1000 [1], but as high as 100 per 1000 in professional footballers [2]. It has been well reported in the literature that there is an increased incidence of early onset osteoarthritis (OA) in patients sustaining an ACL rupture. The development of OA in these often young and active patients can have a devastating effect on their quality of their life and level of activity. It has been acknowledged that the development of early OA is multifactorial and is heavily influenced by associated meniscal tears [3], chondral damage sustained at initial injury [1], and multi-ligament knee injuries [2]. It is currently unclear what effect ACL reconstruction has on the progression of OA. This review will describe the pathophysiology of OA development following ACL rupture, and the effect that an ACL reconstruction has on its development.
AETIOLOGY
Pathophysiology of post-traumatic OA:
There are many factors affecting the development of early OA following ACL rupture. These include trauma to the articular cartilage sustained at the time of injury, changes in gait, age at the time of injury, the presence of associated meniscal tears, and subsequent damage caused by instability of the knee joint leading to abnormal loading of the articular cartilage [1, 4, 5].
ACL injuries are most commonly sustained with the noncontact loading of a valgus knee while twisting in the opposite direction producing a forced internal rotation of the tibia [6]. This impact and subluxation of the joint during ACL rupture causes an impaction injury to the articular cartilage, which has been shown by Potter et al, to be present and MRI detectable in 100% of patients suffering an isolated ACL tear [1]. When an ACL injury is sustained the most common mechanism of noncontact loading of a valgus knee leads to the lateral tibial plateau subluxing anteriorly on the lateral femoral condyl. This causes an impact between the anterior lateral femoral condyl and the posterior lateral tibial plateau. Potter et al., showed, by using morphologic MRI and T2 mapping in 40 patients with 42 isolated ACL ruptures, that the most common areas affected are the lateral tibial plateau and the lateral femoral condyl, as shown in Fig. (1) [1]. They were also able to demonstrate with yearly follow-up MRI’s, that the risk of cartilage loss from these areas was 50 times baseline by years 7-11.
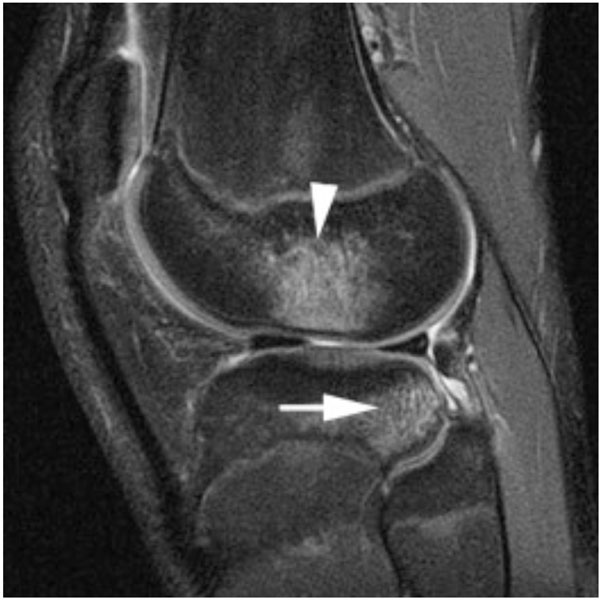 |
Fig. (1). An MRI demonstrating the areas bone oedema caused by an ACL injury. |
This impaction leads to cartilage damage in two phases. Firstly the shear stresses of the impact can separate the cartilage from the underlying bone and cause fissures of varying thicknesses to form. Secondly, this impaction leads to the release of cartilage extra cellular matrix molecules, collagen lattice disruption, and the swelling of the cartilage due to unrestrained negatively charge hydrophilic glycosaminoglycans (GAGs). The levels of GAG has also been found to be lower following an ACL injury, and this correlates directly with the type II collagen content of the articular cartilage [7].
At a cellular level, activation of cytokine and protease cascades occur within the knee joint which increases chondrocyte catabolism [8]. Recent studies [8, 9] have shown that there are a number of pathways involved in initiating progressive chondrocyte degeneration. One pathway is the release of oxygen free radicals from damaged chondrocyte mitochondria, which leads to progressive chondrocyte damage and matrix degradation. Another pathway indicated is the release of fibronectin fragments from impacted cartilage, which stimulates cell damage and matrix degradation. Joint injuries also cause an increase of inflammatory mediators such as tumour necrosis factor alpha (TNF-α), interleukin (IL)-1, nitric oxide, and matrix metalloproteinases (MMPs) within the synovial fluid.
A haemarthrosis, which is usual following an ACL injury, will also have a significant effect on cartilage damage. It has been shown to cause a loss of proteoglycan and inhibit proteoglycan synthesis, as well as inducing chondrocyte apoptosis. The neutrophils in an acute haemarthrosis are activated and produce activated oxygen species, elastase, and lysosomal enzymes, and these degrade proteoglycans [8].
All of these mechanisms lead to cell death in the cartilage. This cell death, initially at the site of the impact, spreads by apoptotic mechanisms to regions of unimpacted cartilage, which leads to expansion of the affected lesion. The consequence of cell death is that it depletes the cartilage of the cells which it requires to repair and maintain the extracellular matrix.
Another important associated factor is age. Although not dependant on the injury itself, it affects the body’s ability to withstand the initial trauma. Articular cartilage chondrocytes show strong evidence of increasing senescence with increasing age. This is alongside the synthesis of smaller more irregular aggrecans, and decreased proteoglycan synthesis, which all reduces the knee’s ability to withstand an impaction injury [10].
Incidence of osteoarthritis following ACL injury:
It is well documented that there is a strong association between OA and ACL injury. What is more variable is the exact incidence of OA following ACL injury, with figures ranging from 10-90% at 10 and 20years after the injury [2, 11]. The mean incidence is about 50% at 20years, which is extremely significant when you consider that most of these patients will be between 20 to 30years of age at the time of ACL injury, and therefore they represent a very large group of young OA patients, which are difficult to manage.
SIGNIFICANCE OF ASSOCIATED INJURY
Impact of associated meniscal tears:
The effect that a meniscal tear has on the development of OA is considerable. Tears are commonly seen in patients suffering from an ACL injury with a reported incidence of about 33% [12]. The mechanisms for this relate to the initial impact injury to the cartilage as described above, and the chronic overload of the joint cartilage generated by the loss of meniscal tissue [13]. The fibrocartilaginous menisci of the knee improve joint stability, load distribution, shock absorption and cartilage lubrication. The type of meniscal tear is important, as this has been shown to affect the development of OA. Longitudinal tears usually occur in association with a definite knee trauma whereas horizontal tears develop in older patients due to degenerative changes in the meniscal tissue [3]. Patients with a degenerative tear are more likely to develop OA with an incidence around 50%, than traumatic tears with an incidence around 33% in isolated meniscal injury [3]. As you would expect the larger the section of meniscus resected at the time of surgery, the higher the incidence of OA, and the poorer the long term functional outcome of the patient [14]. When a meniscal tear is associated with an ACL injury the incidence of OA increases significantly with 50-70% of patients having radiographic changes after 15years [15-20].
Impact of associated bone bruising:
Bone bruising, most commonly identified using magnetic resonance imaging (MRI), can be a sign of compressive chondral injury, even when the chondral surface looks normal during arthroscopy [8]. The location of the bone marrow oedema and therefore bruise, is a reliable indicator of where post traumatic OA is likely to develop in the future. The size of the area of bone marrow oedema is also indicative of the severity and speed of onset of any future OA if it develops [1]. Potter et al., performed a longitudinal study of patients who suffered from ACL injury, some of whom had a reconstruction. At 7 to 11 years post injury, the risk of developing cartilage loss was 50 times that of baseline if a bone bruise was present in the lateral femoral condyle, 30 times if it involved the patella, and 19 times if in the medial femoral condyle. What’s more, ACL reconstruction appeared to provide a chondroprotective affect [1].
Impact of associated cartilage injuries:
A full thickness cartilage lesion that occurs at the time of an ACL injury, as shown in Fig. (2), is not common, with a reported incidence of less than 1% [21]. The effect that this has on recovery from ACL reconstruction and post-operative outcome is debatable. It has been shown in one study that patients who have a full thickness cartilage lesion at the time of an ACL reconstruction, will usually have poorer function in activities of daily living, sports and recreation, quality of life and will experience less improvement following ACL reconstruction [21]. A second study using the same group of patients, with the same scoring systems, found no difference [22]. Both of these studies used the Norwegian National Knee Ligament Registry and the results were based on the same 30 patients that had an isolated ACL rupture and a full thickness cartilage lesion. The only difference that could explain the difference between the two groups would be the timescale. The first by Hjermundrud et al., [22], showed no difference at 1 year follow-up, and the second by Rotterjud et al., [21], showed significant differences using the same outcome measures at 2-5 years. The other authors on the papers were that same, and so publication bias would seem unlikely. It would seem logical that there is some deleterious effect on the knee following a full thickness cartilage lesion occurring alongside an ACL injury, but this effect is likely to be small and should not lead to any changes in current practice [23]. However, this is an area which requires further investigation.
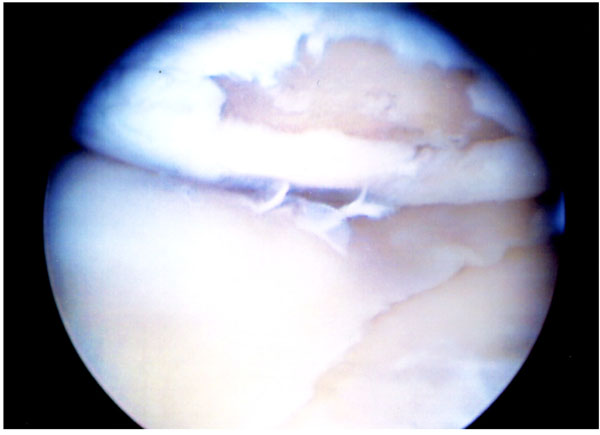 |
Fig. (2). A full thickness cartilage defect following an ACL injury. |
SURGICAL CONSIDERATIONS
Effect of ACL reconstruction on the development of OA:
The effect that ACL reconstruction has on the development of OA, as demonstrated in Fig. (3), is a contentious issue. A Cochrane review in 2005 could not show any conclusive evidence to support ACL reconstruction, and concluded that “there is insufficient evidence from randomised trials to determine whether surgery or conservative management was best for ACL injury in the 1980’s, and no evidence to inform current practice” [24]. Subsequent studies that have shown that an ACL reconstruction can reduce the later incidence of OA compared to no ACL reconstruction, but only if there is good anterior-posterior stability [5, 25]. Contrary to this, other studies have found no protective effects of ACL reconstruction [19].
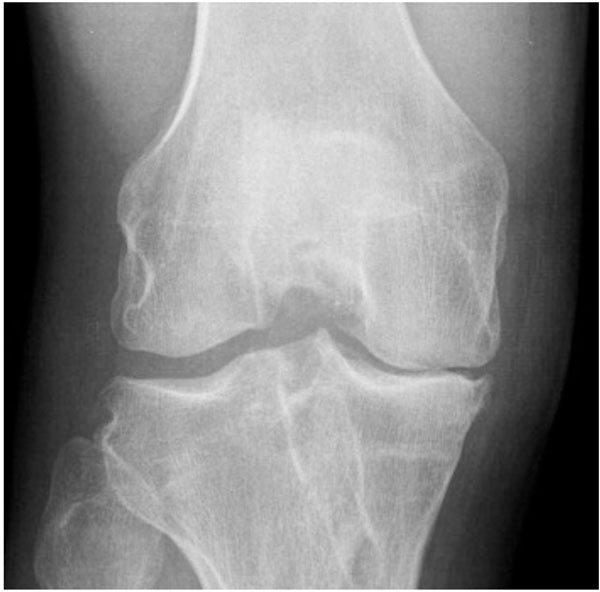 |
Fig. (3). An AP Knee X-ray demonstrating OA following an ACL reconstruction. |
An ACL deficient knee is at higher risk of developing a meniscal tear [26, 27]. As described above, a meniscal tear is a significant risk factor for the early development of OA. It has been shown that if an ACL injury is managed non-operatively initially with a structured rehab regime and the option for delayed reconstruction if required, the incidence of subsequently developing a meniscal tear was significantly higher [28, 29]. Although the same study also showed no difference in overall outcome, 38% required delayed ACL reconstruction due to instability [28].
Choice of graft used in ACL reconstruction:
There are a number of grafts available for ACL reconstructions which include autograft, allograft and synthetic options. The two most popular graft choices, and hence the ones which have received most attention in the literature, are bone-patella tendon-bone (BPTB), and hamstring tendon (HT) graft [12]. There is no definitive evidence that one form of graft is superior to the other in overall outcome [30]. There have been some suggestions that BPTB serves as a catalyst for accelerating the progression of knee OA [29, 31, 32], although this phenomenon has not been shown in all clinical studies [17]. There is also an increased incidence of harvest site symptoms, and kneeling pain in BPTB grafts when compared to HT grafts [31, 32]. Pre clinical animal studies have shown that patella tendon and hamstring tendon grafts are actually stiffer than native ACL’s [33]. This may suggest that these stiffer graft constructs may therefore significantly alter the kinematics of the joint and help in assisting the transmission of increased forces through the rest of the joint, to areas which do not normally receive such high load. This altered biomechanics may also change the biology of the graft construct leading to a sub optimal environment for cells to thrive and produce extra cellular matrix. Therefore, further research is required to establish better, more physiologic graft options with a greater understanding of anatomical graft placement and graft tension.
Timing of ACL reconstruction surgery:
An acute ACL injury will usually result in at least a significant haemarthrosis with significant pain. This leads to a painful stiff knee with decreased mobility in the first few weeks after injury. It has been shown that delaying surgery by about 6 weeks is probably optimal time for reducing the risks of deep vein thrombosis (DVT) [30], without compromising the knee significantly.
There are then two schools of thought ‘early reconstruction and structured rehabilitation’ and ‘structured rehabilitation with delayed reconstruction only if required’. Generally about a third of patients who only have structured rehabilitation later undergo ACL reconstruction due to instability [27]. It has been shown that early ACL reconstruction reduces the incidence of early OA in ACL deficient patients who are intent on continuing activities that involve sidestepping and pivoting activities [34]. It is generally accepted that the rehabilitation that the patient undergoes following an ACL injury is fundamental to their recovery. Shelbourne et al., [35] have shown a relationship between loss of range of motion and OA development in a cohort of 780 consecutive ACL reconstructions at minimum follow up of 5 years. They found that in those who did not have a full range of motion compared to the un-operated limb had a 39% prevalence of OA versus 53% in those who had a less than normal range of motion. The same author has also demonstrated a relationship between loss of range of motion and poor subjective outcome following ACL reconstruction [36]. Therefore, a structured regime organised and run by experienced physiotherapists alongside surgeons as part of a multidisciplinary team approach may result in the best outcomes for patients.
POTENTIAL FUTURE DEVELOPMENTS
The fact that OA development is multifactorial in nature, would suggest that future treatment would require a multimodal strategy. Correction of biomechanical abnormalities such as ACL deficiency will require correction prior to the introduction of any biological solutions. The evidence for biological mediators is growing, even if most of it is in the form of pre-clinical models [8-10].
Reducing apoptosis in the immediate post-impact period can be done using Caspase inhibitors. This could potentially minimise the effect of any localised chondral impact injury by reducing the spread of cell death [8].
Reducing the effects of the inflammatory mediators such as IL–1 can be achieved by drugs like ‘Diacerhein’, which interferes with the inflammatory and catabolic effects of IL-1. TNF-alpha can be inhibited by TNF-alpha receptor fusion proteins, which has been shown to be particularly protective following an ACL injury [8]. Antioxidants such as N-acetylecysteine can reduce the matrix degradation associated with oxygen free radicals, and have been shown to improve proteoglycan content at the impact site [8]. MMPs are one of the enzymes responsible for matrix degradation, and their inhibitors have been shown to reduce GAG loss, but have not proven very effective in reducing post-traumatic OA [8].
The greatest chance for treatment of post traumatic OA in the near future may come in the form of a number of injectable molecules. Bone morphogenetic proteins (BMPs) are potent stimuli of mesenchymal cell differentiation and extracellular matrix formation. BMP-7 has been shown to be particularly effective as a disease-modifying drug in OA. BMP-7 upregulates chondrocyte metabolism and protein synthesis, while preventing chondrocyte catabolism by IL-1 and fibronectin fragments [8]. In an ovine model of femoral condyle impaction injury, it was shown to reduce OA changes in articular cartilage 6 months following impaction [37]. Moore et al., have also shown that fibroblast growth factor 18 (FGF18) can be utilised in a rat model of injury induced OA to modulate the anabolic response of chondrocytes and articular cartilage [38]. As a result, early clinical studies are now underway to treat early OA and cartilage injury.
These developments are exciting, however it will be some time in the future before they become available and common place in clinical practice. In the meantime, simple non operative strategies can be employed to help reduce the impact of OA in the ACL injured population. Obesity [39], lower extremity strength (particularly quadriceps) [40], impact activities and contact sports [41, 42] are all know to be risk factors for developing OA. Adequate counselling should therefore be provided to patients in order to reduce obesity, and increase leg strength. All patients should be aware of the risk associated with returning to participate in at risk impact/contact sports.
SUMMARY
Overall, based on current evidence, early ACL reconstruction using an autologous graft, following the re-establishment of range of movement, combined with a structured rehabilitation regime post operatively is recommended for patients wishing to return to pivoting sports or those with continued instability. The reasons for this are to return a young active patient to full activities to improve their quality of life, to prevent further intra-articular injury such as meniscal tear, and also allow the repair any meniscal injuries at the time of ACL reconstruction surgery. Counter to this is the second haemarthrosis that the patient undergoes, which will have a further deleterious effect on the articular cartilage, and the general risks of orthopaedic lower limb surgery. As yet, there is limited evidence to suggest that early ACL reconstruction will reduce the risk of developing OA in the future.
In the future, a combination of biological mediators will likely play a significant role in preventing the development of early OA following traumatic injury such as ACL rupture. However, the widespread use of these agents will first require large multicentre randomised studies with long term follow up to prove efficacy and achieve appropriate regulatory approval. In the meantime, it is the authors’ belief that the best we can do is to restore the stability and biomechanics of the knee with an ACL reconstruction, to return patients to their desired level of activity and prevent any further meniscal and cartilage injury.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.







