All published articles of this journal are available on ScienceDirect.
Relationship Between Mechanical Properties and Bone Mineral Density of Human Femoral Bone Retrieved from Patients with Osteoarthritis
Abstract
The objective of this study was to analyse retrieved human femoral bone samples using three different test methods, to elucidate the relationship between bone mineral density and mechanical properties. Human femoral heads were retrieved from 22 donors undergoing primary total hip replacement due to hip osteoarthritis and stored for a maximum of 24 hours postoperatively at + 6 °C to 8 °C.
Analysis revealed an average structural modulus of 232±130 N/mm2 and ultimate compression strength of 6.1±3.3 N/mm2 with high standard deviations. Bone mineral densities of 385±133 mg/cm2 and 353±172 mg/cm3 were measured using thedual energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT), respectively. Ashing resulted in a bone mineral density of 323±97 mg/cm3. In particular, significant linear correlations were found between DXA and ashing with r = 0.89 (p < 0.01, n = 22) and between structural modulus and ashing with r = 0.76 (p < 0.01, n = 22).
Thus, we demonstrated a significant relationship between mechanical properties and bone density. The correlations found can help to determine the mechanical load capacity of individual patients undergoing surgical treatments by means of noninvasive bone density measurements.
1. INTRODUCTION
Information on bone material properties can be useful for individualised surgical treatment, e.g., by adapting the implant design preoperatively in order to optimise the interface between the bone and implant. Furthermore, the relationship between mechanical bone properties and bone mineral density (BMD) [1-5] of the human femoral head is a clinically important step towards predicting the risk of femoral neck fracture in a patient. In an avascular necrosis of the femoral head, the application of electromagnetic fields for stimulation of bone regeneration is used as a femoral head-preserving therapy [6]. Knowledge of mineral density and the mechanical properties of the femoral bone could be useful for patient-specific treatment in order to obtain adequate distribution of the electromagnetic fields within the relevant bone region. For example, the bipolar induction screw system (BISS) [6] is used as an electromagnetic implant for recalcification and remineralisation of bone defects in osteonecrosis and fracture healing.
Extensive experimental investigations have shown that different sample geometries [7,8] and sample positions [9-11] influence the measured properties of the material. Human bone structure is able to adapt to mechanical loading, e.g., the adaptation of the trabecular bone to mechanical stress based on "Wolff's Law of Transformation" [12]. Macroscopically, long bones are divided into two different regions: the cortical bone and the cancellous bone. The microstructure of cancellous bone consists of trabeculae and bone marrow [13].
To predict fracture risk, the estimation of BMD of human femoral necks is extensively used [14]. The BMD can be determined through various methods, which can serve the diagnosis and therapy monitoring of osteoporosis [15, 16]. In general, radiographic analysis is used, i.e., dual energy X-ray absorptiometry (DXA) or quantitative computed tomography (QCT) is applied for assessing patients’ BMD. Furthermore, mineral density of bone samples can be determined experimentally ex-vivo by ashing [17-19]. DXA and QCT are standard diagnostic methods, while ashing is regarded as the gold standard of current bone mineral densitometry techniques.
To determine the structural modulus or ultimate compression strength of human cancellous bone, uniaxial compression tests have been used in various studies [3, 8, 10, 19-22]. Experimental mechanical investigations are needed to evaluate numerical simulations involving bone tissue. The relationship between ultimate compression strength, DXA, QCT and ashing was determined by Ebbesen et al. [23] for the lumbar vertebral body. To calculate the fracture risk of the femoral head, it is necessary to investigate bone material from the femoral head using different techniques.
The aim of the present experimental study was to analyse human cancellous bone samples obtained from the femoral heads of caucasian patients undergoing primary total hip replacement (using DXA, QCT and ashing) and elucidate the relationship between BMD and mechanical properties (structural modulus and ultimate compression strength).
2. MATERIALS AND METHODS
Human femoral heads were retrieved from 11 male and 11 female donors aged between 51 and 84 years undergoing primary total hip replacement. The femoral heads were stored for a maximum of 24 hours postoperatively at + 6 °C to 8 °C. Subsequently, these were frozen in small sealed containers at - 20°C [24]. The specimens were moved twelve hours before each testing to the refrigerator (+ 6 °C to 8 °C) and frozen at - 20 °C between the different tests. DXA and QCT investigations were performed first, followed by the analysis of the mechanical properties and finally by ashing of the bone. All tests were conducted at room temperature. The tests were approved by the local ethical committee of the University of Rostock (A 2009 38).
2.1. Sample Preparation
To avoid buckling of the test samples during the uniaxial compression tests, cylindrical bone samples with a height to diameter ratio between 1 and 2 were used according to DIN 50106 [25]. Therefore, a diamond hollow drill (Günther Diamantwerkzeuge, Idar-Oberstein, Germany) was positioned on the femoral head at the base of the ligamentum capitis femoris (Fig. 1) and aligned with the femoral neck axis [13]. Consequently, one cylindrical sample of approximately 30 mm in length and 12 mm in diameter was cut out of each femoral head. The endplates of bone cylinders were prepared in a tube-like template [20] to assure their parallel alignment and exact length of 15 mm.
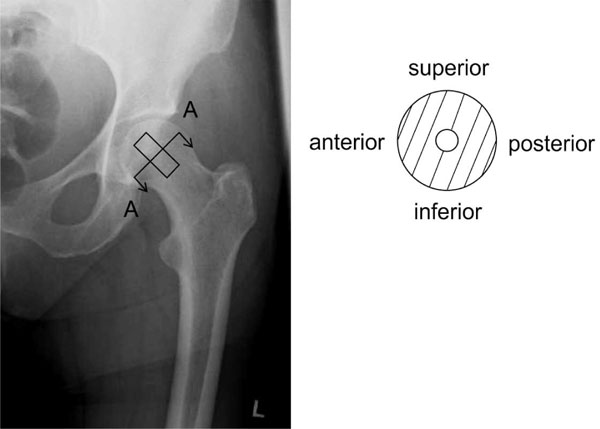
Sample orientation in anterior-posterior X-ray (left) and schematically sectional drawing A-A (right). The samples were drilled out of the centre of the human femoral head.
2.2. Bone density measurements
BMD was analysed using two different methods, DXA and QCT. Furthermore, it was assessed by ashing. The bone samples used in this study only consisted of cancellous bone. Hence, all data obtained are representative for cancellous bone.
2.2.1. DXA
DXA is a two-dimensional method to determine bone density using two slightly different energy X-ray sources. The BMD measurements of the cylindrical test samples were conducted with a DXA device (Lunar Prodigy, General Electric (GE) Healthcare, Munich, Germany) using the research option (electrical potential of 76 kV and electrical current of 0.15 mA). Calibration of the device was performed using a cuboid calibration phantom (200 x 130 x 60 mm) with three bone equivalent chambers, each with a different BMD value (0.5 g/cm², 1.0 g/cm² and 1.5 g/cm²). The BMD was obtained by setting a region of interest (ROI) at the femoral neck using the DXA software enCORE™ 2007 (version 11.40.004, General Electric (GE) Medical Systems, Madison, WI, USA). DXA is clinically used for measuring BMD, predicting the risk of osteoporotic fractures, effectiveness of anti-osteoporotic therapy, as well as evaluating the periprosthetic bone loss after total joint replacement and the bone remodeling process [26-28].
2.2.2. QCT
A QCT (Aquilion 64, Toshiba Medical Systems, New York, USA) was used for determining three-dimensional bone density. For this, a bone phantom (Toshiba Medical Systems, New York, USA) with five reference chambers containing materials of five different densities was placed alongside the cylindrical bone samples. The QCT determines the density of the samples by correlating to the reference densities of the phantom. The Osteo CT setting of the pelvis was used and the scanning parameters were 120 kV, 50 mA, one rotation time and a range of 8 mm. After the QCT measurement, a cross-sectional area near the middle of the samples was chosen to analyse bone density.
2.2.3. Ashing
The cylindrical bone samples were combusted in a tube furnace (Nabertherm, Lilienthal, Germany) after the uniaxial compression test at 800 °C for 5 h [29]. The remaining ash represented the BMD of the sample, which was determined using equation 1 [17,29] based on the original bone sample volume.
where ρbone represents the apparent bone density, Mi the ash mass and Vo the original volume of the bone sample.2.3. Uniaxial Compression Test
Before ashing, a uniaxial compression test to determine the structural modulus and the ultimate compression strength was carried out. The structural modulus (ES) provides a linear relationship between stress (σ) and strain (ε) according to Hooke’s law (equation 2). Stress and strain data were obtained from the linear section of the load-displacement curve. The ultimate compression strength (σmax) described the point of maximum compression stress applied on the bone samples before failure during the test. The cylindrical bone samples were investigated using a universal testing machine (Z050, Zwick/Roell, Ulm, Germany). Compression
load was applied at a velocity of 5 mm/min [23] until a total displacement of 4 mm was reached.
2.4. Statistical Analysis
Pearson correlation was used to determine the linear correlation coefficient (r) between the different methods of measuring BMD and mechanical properties of the human bone samples. Statistical analysis was determined with the software SPSS, Version 20 (SPSS Inc., Chicago, USA). All p-values were two-sided and p<0.05 was considered significant.
3. RESULTS
3.1. Data of BMD and Mechanical Parameters
Within the different methods used for the measurement of BMD, high standard deviations were observed. The mean and standard deviations of BMD and mechanical properties are shown in Table 1. DXA analyses of the 22 bone samples measured an average BMD of 385±133 mg/cm². Using QCT, an average BMD of 353±172 mg/cm³ was determined. Ashing measured a BMD similar to that obtained by QCT, with an average of 323±97 mg/cm³. The determination of the structural modulus and the ultimate compression strength averaged 232±130 N/mm² and 6.1±3.3 N/mm², respectively. The comparison of 11 female and 11 male bone samples showed nearly the same range of mean values for DXA, QCT and ashing. In general, smaller standard deviations were found in the male bone samples.
Mean Values±Standard Deviations of Mechanical Properties and BMD (nf = 11 Female Donors, nm = 11 Male Donors)
| Cancellous Bone Samples | Structural Modulus [N/mm2] | Ultimate Compression Strength [N/mm2] | DXA [mg/cm2] | QCT [mg/cm3] | Ashing [mg/cm3] |
|---|---|---|---|---|---|
| n = 22 | 232±130 | 6.1±3.3 | 385±133 | 353±172 | 323±97 |
| nf = 11 | 253±168 | 5.6±3 | 381±167 | 355±210 | 319±123 |
| nm = 11 | 211±80 | 6.6±3.7 | 390±97 | 351±134 | 327±69 |
3.2. Comparison of DXA, QCT, Ashing and Mechanical Parameters
Comparison between the different methods of BMD analysis demonstrated the best significant linear correlation between ashing and DXA (r = 0.89, p < 0.01) (Fig. 2). Correlation of QCT to the structural modulus resulted in r = 0.73 (p<0.01). There was a significant linear correlation between the structural modulus and ash bone density of r = 0.76 (p<0.01), as well as between ultimate compression strength and an ash bone density of r = 0.73 (p<0.01), whereas lower correlations between the mechanical properties and DXA (Table 2) were obtained. However, there was no linear correlation between the age of the 22 patients (70.6±9.4 years) and the structural modulus, as well as with the ultimate compression strength, DXA, ashing and QCT.
Calculated Coefficients (r) for Linear Correlation Between the Mechanical Parameters and Measured Bone Densities of the 22 Cylindrical Samples
* Correlation is significant at the 0.05 level (2-tailed)
** Correlation is significant at the 0.01 level (2-tailed).
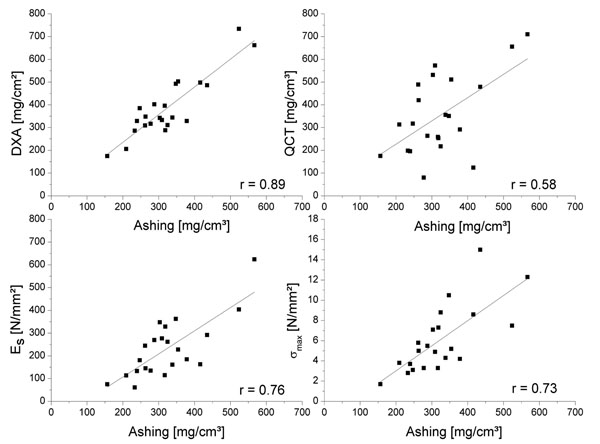
Linear correlation of BMD measured by different methods (DXA and QCT) and mechanical properties, i.e., structural modulus (Es) and ultimate compression strength (σmax) compared to ashing.
4. DISCUSSION
The aim of the present study was to compare different methods for measuring human BMD and to correlate the results with the mechanical properties of retrieved cancellous bone samples. Using DXA, QCT, ashing and uniaxial compression tests, 22 cylindrical bone samples from femoral heads retrieved from patients with osteoarthritis were investigated. Linear correlations were found between mechanical properties and the BMD of the bone samples, whereas high standard deviations within the different test data as well as differences between the various methods used for bone density measurements were found. In several studies, BMD and material parameters of human cancellous femoral bone samples retrieved post-mortem were calculated [20-22,30]. Samples from osteoarthritic and osteoporotic patients, as well as from healthy donors post mortem, showed significant differences concerning mechanical properties [31]. The BMD of osteoarthritic samples was reduced.
The experimental study of Cody et al. [30] using DXA demonstrated a BMD at the femoral neck of 650±130 mg/cm², which is roughly twice the BMD determined in our study. Sierpowska et al. [21] measured a lower BMD with 237±66 mg/cm³ by means of DXA. The volumetric BMD was calculated by dividing the areal BMD with the thickness of the bone samples [21]. The samples were retrieved from the distal femur post mortem and prepared in a bandage of moistened saline.
Furthermore, Cody et al. [30] analysed bone samples from the cancellous femoral neck using QCT and obtained an average bone density of 1056±33 mg/cm³. Steinhauser et al. [20] measured a mean value of 222.3±134.4 mg/cm³, which is within the QCT range of the present study. The bone samples used for our study were only taken from patients suffering from severe osteoarthritis of the femoral head, i.e. low BMD could occur more frequently than in random post mortem bodies. Moreover, bone samples were derived from the femoral head in contrast to other studies analyzing samples from the femoral neck [20,30]. In avascular head necrosis, the implants for electromagnetic bone stimulation are placed in the femoral head. Hence, the region of interest was cancellous bone of the femoral head in the present study. Furthermore, a sufficient portion of cancellous bone using retrieved femoral heads in the case of total hip arthroplasty could not be achieved at the femoral neck region. The comparison of the different methods to measure BMD was made. An exact method to determine BMD is ashing. Rohlmann et al. [22] obtained an average mineral ash density of 392±130 mg/cm³ for cancellous bone from human femoral head post mortem. Öhman et al. [19] found an average ash density of 300±70 mg/cm³ of cancellous bone samples from the human femoral head, which were stored in a 70% ethanol solution. Both experimental studies (Table 3) produced results consistent with ours.
| DXA [mg/cm2] | QCT [mg/cm3] | Ashing [mg/cm3] | Es [N/mm2] | σ max [N/mm2] | |
|---|---|---|---|---|---|
| Rohlmann et al. 1980 p.m.1 [22] | - | - | 399.2±130 | 389.3±270.1 | 7.3±4.0 |
| Cody et al. 1999 p.m.1 [30] | 650±130 | 1056±33 | - | - | - |
| Steinhauser et al. 2006 p.m.1 [20] | - | 222.3±134.4 | - | 385.7±189.4 | 8.5±6.0 |
| Sierpowska et al. 2005 p.m.1 [21] | 237±66* | - | - | 624.4±213.9 | 10.9±4.2 |
| Öhman et al. 2007 fixed2 [19] | - | - | 300±70 | 273±106 | 18±6.4 |
| Own data1 | 385±133 | 353±172 | 323±97 | 232±130 | 6.1±3.3 |
1 p.m = post mortem
2 fixed = fixation with 70% ethanol
* BMD expressed as mg/cm3
Material properties of bone samples are often associated with Young’s modulus [8,20,21,32]. Our samples were prepared in a single orientation, so that the distribution of the Young’s modulus was not due to anisotropy [3]. Since bone is a complex material made of different organic and inorganic components, however, the nomenclature “structural modulus” (ES) describes Young’s modulus (mechanical properties) more accurately in relation to bone samples [33]. Sierpowska et al. [21] found a high average Young’s modulus of 624.4±213.9 N/mm² of cancellous bone and ultimate compression strength of 10.9±4.2 N/mm² (specimen geometry: cylindrical Ø16 mm x 8 mm) using human distal femoral bone, whereas Steinhauser et al. [20] determined values of 385.7±189.4 N/mm² for Young’s mod-ulus and 8.5±6.0 N/mm² for ultimate compression strength (specimen geometry: cylindrical Ø7.2 mm x 14.4 mm). Öhman et al. [19] found the highest ultimate compression strength (Table 3). Birnbaum et al. [8] demonstrated results of the right and left proximal hip joint samples of 146 N/mm² (minimum) to 320 N/mm² (maximum) for Young’s modulus and 3.7 N/mm² (minimum) to 7.85 N/mm² (maximum) for ultimate compression strength (sample geometry: cylindrical Ø11 mm x 24 mm).
Schröter [32] determined an average Young’s modulus of 206.13±41 N/mm² for cylindrical samples (diameter 10 mm, length 15 mm). This corresponds to the average structural modulus obtained in our study. Different sample geometries and sample positions are possible reasons for the high variations in the results among the different research groups. Keaveny et al. [7] observed that both modulus and strength could depend on the specimen geometry when using standard techniques for compression testing. They proposed that test cylinders with a height to diameter ratio of 2:1 proved advantageous over cubes [7]. In contrast to this investigation our cylindrical bone samples with a height to diameter ratio between 1 and 2 were used according to DIN 50106 [25].
Bone quality depends on geometric and material factors with regard to fracture risk [34]. The investigated material in this examination was bone of the femoral head retrieved from osteoarthritic patients in contrast to the human post mortem material used in various other studies [20-22, 30]. Bone tissue retrieved post mortem may reveal varying material properties compared to samples from osteoarthritic patients. In our present study bone samples from the femoral head of osteoarthritic patients were tested in order obtain data from fresh cancellous bone. Hence, analysis of the femoral neck and prediction of the fracture risk were not focused.
To obtain valid data for the mechanical properties of bone material structures from different density measuring methods, a high correlation coefficient is required. DXA and QCT measurements showed moderate correlation with the structural modulus and the ultimate compression strength. The results of Snyder and Schneider [1] for cortical samples of human tibiae showed lower correlation between Young’s modulus and bone density calculated by CT. In contrast to conventional CT, the QCT method determines the physical density as mass / volume of each voxel more precisely. The highest correlation in the present study was obtained between structural modulus and ashing. However, ashing cannot be applied to patient´s measurements, in contrast to DXA or QCT.
In summary, a relationship between the mechanical properties and the mineral density of cancellous bone was observed. The linear correlations found between mechanical data and BMD can help to determine the mechanical load capacity of individual patients in case of surgical treatments by means of non-invasive bone density measurements. In terms of total joint replacement the knowledge of the bone mineral density can help to derive biomechanical properties of the bone stock in order to select adequate implant designs intraoperatively, e.g. the type of fixation with or without bone cement, as well as to estimate stress shielding within the bone stock postoperatively. Furthermore, the bone regeneration and remodeling process around endoprosthetic and electromagnetic implants could be evaluated postoperatively.
The mechanical parameters determined in our study were lower than those reported in the literature. The differences could result from different retrieval areas and storage of the bone samples. Further investigations should be undertaken to clarify the discrepancies of BMD data between the different measurement techniques and research groups, i.e., standardised measurements of mechanical properties as well as the source, orientation and size of bone samples need to be addressed. Knowledge of individual bone material properties obtained by non-invasive bone density measurements (e.g., DXA or QCT) could be useful for treatment, e.g., in the application of electromagnetic fields for bone regeneration and joint arthroplasty by adapting the implant preoperatively in order to optimise the interface between the bone and the endoprosthetic implant.
ACKNOWLEDGEMENTS
We would like to thank Prof. Dr. M. Köckerling and A. Bernsdorf at the Institute of Chemistry, Inorganic Solid State Chemistry Group, supporting the use of the tube furnace, Prof. Dr. K. Hauenstein, Department of Diagnostic and Interventional Radiology, University Medicine Rostock, for technical support concerning QCT and Deutsche Forschungsgemeinschaft (DFG) for supporting the graduate project (GRK 1505/1 welisa).
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.


