RESEARCH ARTICLE
BMP-2 Dependent Increase of Soft Tissue Density in Arthrofibrotic TKA
Tilman Pfitzner*, 1, Eric Röhner1, Veit Krenn2, Carsten Perka1, Georg Matziolis1
Article Information
Identifiers and Pagination:
Year: 2012Volume: 6
First Page: 199
Last Page: 203
Publisher ID: TOORTHJ-6-199
DOI: 10.2174/1874325001206010199
Article History:
Received Date: 4/2/2012Revision Received Date: 13/4/2012
Acceptance Date: 22/4/2012
Electronic publication date: 16/5/2012
Collection year: 2012

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Arthrofibrosis after total knee arthroplasty (TKA) is difficult to treat, as its aetiology remains unclear. In a previous study, we established a connection between the BMP-2 concentration in the synovial fluid and arthrofibrosis after TKA. The hypothesis of the present study was, therefore, that the limited range of motion in arthrofibrosis is caused by BMP-2 induced heterotopic ossifications, the quantity of which is dependent on the BMP-2 concentration in the synovial fluid.
Eight patients with arthrofibrosis after TKA were included. The concentration of BMP-2 in the synovial fluid from each patient was determined by ELISA. Radiologically, digital radiographs were evaluated and the grey scale values were determined as a measure of the tissue density of defined areas. Apart from air, cutis, subcutis and muscle, the soft-tissue density in the area of the capsule of the suprapatellar pouch was determined. The connection between the BMP-2 concentration and the soft-tissue density was then investigated.
The average BMP-2 concentration in the synovial fluid was 24.3 ± 6.9 pg/ml. The density of the anterior knee capsule was on average 136 ± 35 grey scale values. A linear correlation was shown between the BMP-2 concentration in the synovial fluid and the radiological density of the anterior joint capsule (R=0.84, p = 0.009).
We were able to show that there is a connection between BMP-2 concentration and soft-tissue density in arthrofibrosis after TKA. This opens up the possibility of conducting a prophylaxis against arthrofibrosis in risk patients by influencing the BMP-2 pathway.
INTRODUCTION
Arthrofibrosis after total knee arthroplasty (TKA), the incidence of which is reported as 3-10% in the literature [1-4], remains an unsolved problem. As a result of the permanent painful limited range of motion, many patients experience a pronounced functional impairment of the affected knee, despite the TKA having been implanted correctly. Although risk factors such as the preoperative range of motion, previous surgery or diabetes mellitus are identified [3-5], the precise aetiology has remained unclear to date [3, 6-8]. This means that therapy can only be symptom-oriented at present, ranging from arthroscopic and open arthrolysis to revision arthroplasty [2, 3, 9, 10]. However, regardless of the procedure used, the results are largely unsatisfactory [3, 9].
Without a clear pathomechanism, it has not been possible to develop a prophylaxis against arthrofibrosis up to now. However, cytokines in the synovial fluid, in particular bone morphogenetic protein 2 (BMP-2), appear to play a central role in the pathogenesis of arthrofibrosis. In a previous study, we demonstrated elevated concentrations of BMP-2 in the synovial fluid in arthrofibrosis after TKA [11]. Apart from its inflammatory effect, BMP-2 is known in particular as a potent inductor of heterotopic ossifications (HO) in tissue [12-15]. Not only fibrosis of the soft tissues but also an increased incidence of HO were thus observed in further studies on arthrofibrosis after TKA [1, 16-19].
In the future, it might be possible to reduce HO in the periarticular soft tissue by influencing the BMP-2 pathway and thus provide a prophylaxis against arthrofibrosis in risk patients, comparable to the established ossification prophylaxis in hip arthroplasty [20, 21].
The hypothesis in the present investigation was therefore that the limited flexion in arthrofibrosis after TKA is caused by HO in the anterior soft tissue, the quantity of which depends on the BMP-2 concentration in the synovial fluid.
MATERIALS AND METHODOLOGY
Patients with primary arthrofibrosis after TKA were included in this retrospective study. All of the study patients are part of a patient population of a previous study on BMP-2 expression in synoviocytes in patients with arthrofibrosis after TKA [11]. The study protocol was approved by the institutional review board and all patients gave their informed consent. Age, sex, side and period since primary implantation were documented for each patient.
Up to now, the literature has not included a uniform definition of arthrofibrosis after TKA [3]. In the present study protocol, arthrofibrosis was defined as a painful limitation of flexion < 90° and an extension deficit > 10°, according to the classification of Yercan [22]. A pain duration of at least 6 months since implantation, a resting pain of > 5 and a weight bearing pain of > 7 on a visual analogue scale (VAS) were also defined as inclusion criteria regarding pain.
If there were signs of secondary arthrofibrosis present like infection, loosening, instability, impingement or malalignment, these knees were excluded. In all patients the same diagnostic algorithm was used. Every patient was clinically investigated by the senior author. At this point instability in the coronal (collateral ligamants) or sagittal plane (PCL) was detected. After that whole leg standing a.p. radiographs and lateral radiographs for exclusion of loosening, component malalignment and static impingement due to component oversizing were performed in all cases. For exclusion of infection all patients received blood sample analysis for blood leukocyte count, CRP and ESR such as joint fluid aspiration with following microbiological testing (14 days incubation). A pathological finding in one or more investigation lead to the exclusion of the patient.
Joint fluid was collected via aspiration in all knees once during the study. A minimum of 5 ml of synovial fluid was immediately cryoconserved and stored at -20°C until examination. The measurement of the concentration of BMP-2 was performed with ELISA.
BMP-2 ELISA
The "Quantikine BMP-2 Immunoassay Kit" was used for analysing the concentration of BMP-2 in the synovial fluid according to the manufacturer's protocol (R&D systems, Minnesota, USA). The technique of this assay is a quantitative sandwich enzyme immunoassay. Extinction was measured in a microplate reader at a wavelength of 450nm.
Radiological Investigation
The lateral digital radiographs of each patient, which were done within the context of routine clinical diagnostics, were evaluated according to standardised procedures. Of the original 10 patients, 2 had to be subsequently excluded due to the lack of digital radiographs. The digital images of the remaining 8 patients were evaluated using image processing software (ImageJ 1.45l, Maryland, USA). The individual grey scale values of defined points were recorded as a measure for the density of the tissue [23].
In order to examine the interindividual comparability of the images, the grey scale values of standardised measuring points (air, cutis, subcutis, gastrocnemius muscle) were first compared using the image processing software.
For qualitative assessment of the soft-tissue density in the joint capsule / HO, the grey scale values in the area of the joint capsule of the suprapatellar pouch were evaluated. This was done by measuring a “region of interest” with a defined measuring square of 24mm2 (Fig. 1).
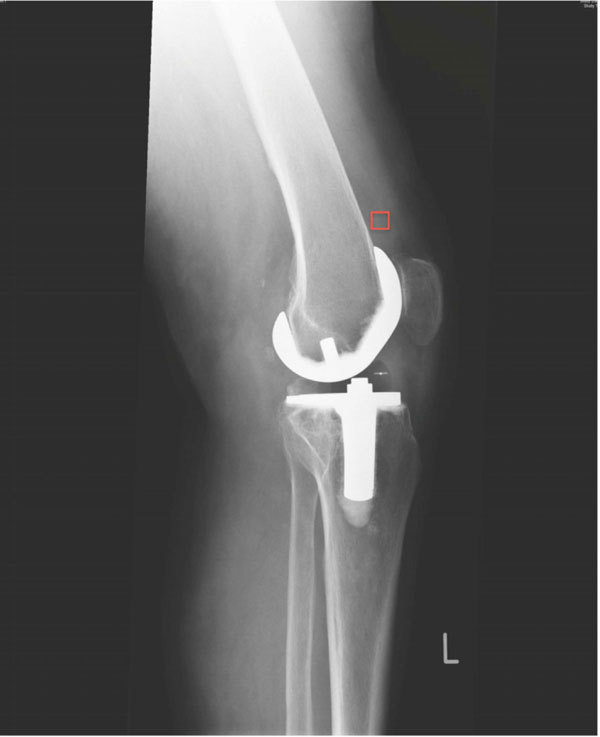 |
Fig. (1). Standard digital radiographs with region of interest measurement. |
Statistics
Descriptive statistics (mean, minimum, maximum and standard deviations) were calculated from the measured data. Correlation analysis was done with the Pearson test. P=0.05 was defined as the level of significance.
RESULTS
The average age of the 5 male and 3 female patients included was 67.5 ± 9.4 years. The side distribution of left to right knees showed a ratio of 7:1. The duration since primary implantation was on average 26.6 ± 15.7 months.
BMP-2 ELISA
The concentration of BMP-2 in the synovial fluid of the analysed patients with arthrofibrosis after TKA was 24.3 ± 6.9 pg/ml.
Radiological Investigation
The evaluation of the digital, lateral radiographs showed only slight differences in the grey scales of the reference tissue, so that the subsequent evaluation of the anterior soft tissues was possible without correction in the sense of a standardisation. The grey scale values of air were on average 2 ± 1.4. The cutis showed average grey scale values of 10.9 ± 2.7, the subcutis 26.9 ± 8.4 and the gastrocnemius muscle 69.8 ± 11.6 (Fig. 2).
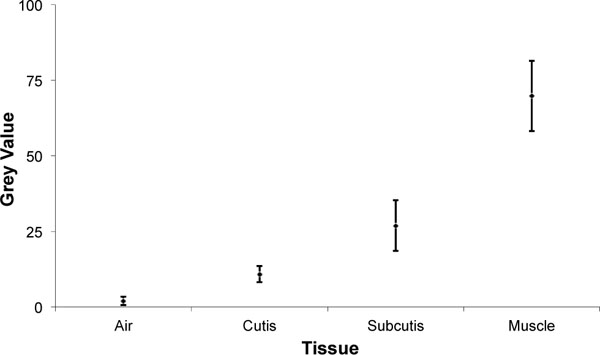 |
Fig. (2). Grey values of different measured tissues. |
Correlation Analysis Between BMP-2 Concentration and Soft-Tissue Density
The grey scale values of the measuring square in the area of the anterior knee capsule were on average 136.2 ± 34.5.
In the correlation analysis, a close linear correlation was found between the concentration of BMP-2 in the synovial fluid and the grey scale values of the anterior joint capsule as a measure of tissue density (R=0.84, p = 0.009) (Fig. 3).
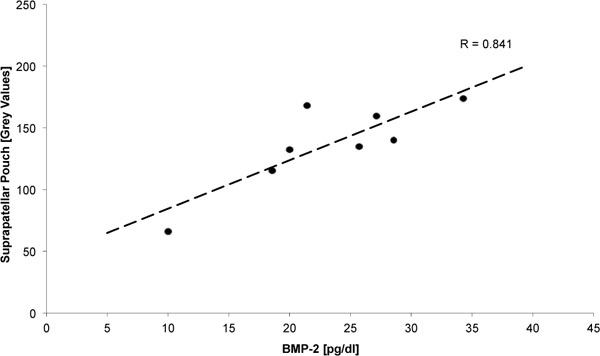 |
Fig. (3). Linear correlation between BMP-2 concentration and Density of the suprapatellar pouch. |
DISCUSSION
In this investigation, we were able to show that there is a connection between BMP-2 concentration in the synovial fluid and soft-tissue density of the anterior joint capsule in patients with arthrofibrosis after TKA. Although this increased density could be differentiated radiologically (Fig. 4), it did not reach the density of bone, so that radiologically visible HO were not present in any of the cases.
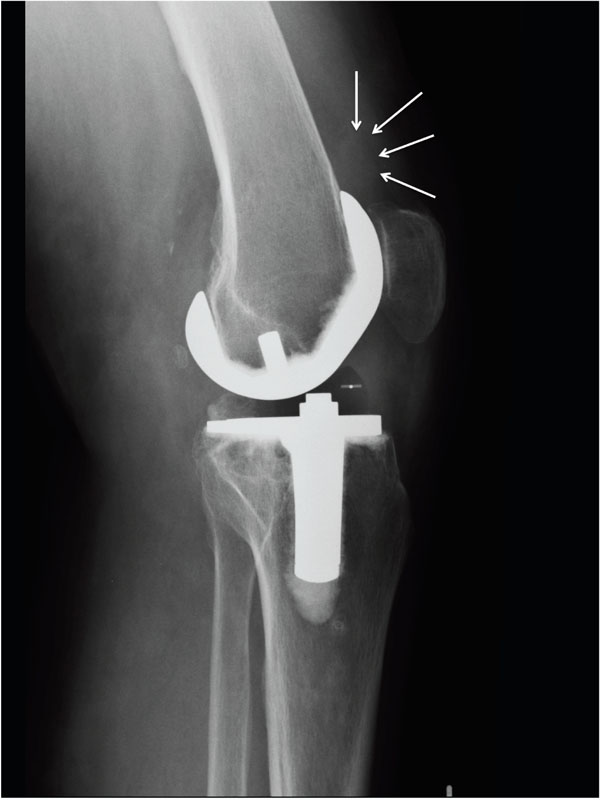 |
Fig. (4). Radiodense soft tissue formation at the suprapatellar pouch. |
This is consistent with the observations of Freeman et al. [17]. On the basis of micro-CT and histology of arthrofibrosis after TKA, they demonstrated mineralised areas in the soft tissue with a density equivalent to bone, which were not identifiable as ossifications in conventional radiographs. The authors suspected the cause to be the insufficient resolution of conventional radiographs to reveal the ossifications present. However, the amount of ectopic bone in the soft tissue correlated with the extent of the patients' limitation of range of motion [17]. This leads to the conclusion that HO may already be present histologically, with clinically relevant impairment of flexion, although they are not detectable in conventional radiographs.
In the pathogenesis of arthrofibrosis, the persistence of physiological inflammation leads to a transdifferentiation of fibroblasts [1, 7, 17, 24, 25], followed by fibrosis of the soft tissue [1, 18, 19, 26]. Within the context of this tissue transformation, further studies have demonstrated HO in arthrofibrosis [1, 16-19].
A connection between BMP-2 concentration in the synovial fluid and HO in arthrofibrosis after TKA has not been described in the literature to date.
In a previous study, we showed an elevated BMP-2 concentration in the synovial fluid (24.3 ± 6.9 pg/mL) and upregulation of BMP-2 in the synoviocytes in arthrofibrosis after TKA. The control group of non-operated knees showed a significantly lower BMP-2 concentration of 5.9 ± 4.8 pg/mL [11]. However, a “normal” range of BMP-2 concentration in the synovial fluid has not been described before.
Apart from its proinflammatory function, BMP-2 is also a strong inductor of heterotopic ossifications [11-15]. In vitro, it causes the osteogenic differentiation of human mesenchymal stem cells [27]. Within the context of a prolonged inflammation in arthrofibrosis, BMP-2 may therefore induce these HO in the soft tissue [28]. Corresponding to this, the upregulation of BMP-2 and BMP-9 has been shown in patients with HO after hip arthroplasty [29, 30].
In clinical application, chronic painful inflammations with pronounced HO have been observed after the application of BMP-2 in spondylodeses [31-33] and in fracture healing [34].
One limitation of the present study is the small number of cases, resulting from the low rate of primary arthrofibroses
after TKA without any secondary cause. This low number of patients corresponds to a low power of this study, i.e. it is not possible to exclude a correlation between BMP-2 and soft tissue density if it had not been detected. But based on the statistically significant correlation even the low number of patients is sufficient to show a link between BMP-2 and soft-tissue density. A further limitation of this study is the absence of histological examinations of the altered joint capsule tissue. This was not possible in retrospect, due to the retrospective design of the study.
CONCLUSION
With the present study, we have shown a connection between the BMP-2 concentration in the synovial fluid and soft-tissue density in the area of the anterior joint capsule in arthrofibrosis after TKA. In the future, it may be possible to influence the BMP-2 pathway as an inductor of HO in risk patients as a prophylaxis against arthrofibrosis after TKA.
CONFLICT OF INTEREST
Declared none.
ACKNOWLEDGEMENTS
The current study was supported by a grant from the “Deutsche Arthrose-Hilfe e.V.” (German Arthritis Foundation). This funding did not influenced the development of the study, the scientific work or the content of this manuscript. The authors declare that there are no conflicts of interest and no further relevant financial relationsships to disclosure.
REFERENCES
| [1] | Freeman TA, Parvizi J, Della Valle CJ, Steinbeck MJ. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty Fibrogenesis Tissue Repair 2009; 2(1): 5. |
| [2] | Gollwitzer H, Burgkart R, Diehl P, Gradinger R, Buhren V. Therapy of arthrofibrosis after total knee arthroplasty Orthopade 2006; 35(2 ): 143-52. |
| [3] | Schiavone Panni A, Cerciello S, Vasso M, Tartarone M. Stiffness in total knee arthroplasty J Orthop Traumatol 2009; 10(3 ): 111-8. |
| [4] | Gandhi R, de Beer J, Leone J. Predictive risk factors for stiff knees in total knee arthroplasty J Arthroplasty 2006; 21(1 ): 46-52. |
| [5] | Ritter MA, Harty LD, Davis KE, Meding JB, Berend ME. Predicting range of motion after total knee arthroplasty. Clustering, log-linear regression, and regression tree analysis J Bone Joint Surg Am 2003; 85-A(7 ): 1278-85. |
| [6] | Skutek M, Elsner HA, Slateva K, et al. Screening for arthrofibrosis after anterior cruciate ligament reconstruction: analysis of association with human leukocyte antigen Arthroscopy 2004; 20(5 ): 469-73. |
| [7] | Watson RS, Gouze E, Levings PP, et al. Gene delivery of TGFbeta1 induces arthrofibrosis and chondrometaplasia of synovium in vivo Lab Invest 2010; 90(11 ): 1615-27. |
| [8] | Jerosch J, Aldawoudy AM. Arthroscopic treatment of patients with moderate arthrofibrosis after total knee replacement Knee Surg Sports Traumatol Arthrosc 2007; 15(1 ): 71-. |
| [9] | Su EP, Su SL, Della Valle AG. Stiffness after TKR: how to avoid repeat surgery Orthopedics 2010; 33(9 ): 658. |
| [10] | Fitzsimmons SE, Vazquez EA, Bronson MJ. How to treat the stiff total knee arthroplasty? a systematic review Clin Orthop Relat Res 2010; 468(4 ): 1096-6. |
| [11] | Pfitzner T, Geissler S, Duda G, Perka C, Matziolis G. Increased BMP expression in arthrofibrosis after TKA Knee Surg Sports [Epub ahead of print]. Available at http://www.springerlink.com/content/830lp164u64086mh/Traumatol Arthrosc 2011. |
| [12] | Yoshikawa H, Yoshioka K, Nakase T, Itoh K. Stimulation of ectopic bone formation in response to BMP-2 by Rho kinase inhibitor: a pilot study Clin Orthop Relat Res 2009; 467(12 ): 3087-95. |
| [13] | Klammert U, Nickel J, Wurzler K, et al. Biological activity of a genetically modified BMP-2 variant with inhibitory activity Head Face Med 2009; 5: 6. |
| [14] | Zeckey C, Hildebrand F, Frink M, Krettek C. Heterotopic ossifications following implant surgery-epidemiology therapeutical approaches and current concepts Semin Immunopathol 2011; 33(3 ): 273-86. |
| [15] | Toom A, Arend A, Gunnarsson D, et al. Bone formation zones in heterotopic ossifications: histologic findings and increased expression of bone morphogenetic protein 2 and transforming growth factors beta2 and beta3 Calcif Tissue Int 2007; 80(4 ): 259-67. |
| [16] | Daluga D, Lombardi AV Jr, Mallory TH, Vaughn BK. Knee manipulation following total knee arthroplasty. Analysis of prognostic variables J Arthroplasty 1991; 6(2 ): 119-28. |
| [17] | Freeman TA, Parvizi J, Dela Valle CJ, Steinbeck MJ. Mast cells and hypoxia drive tissue metaplasia and heterotopic ossification in idiopathic arthrofibrosis after total knee arthroplasty Fibrogenesis Tissue Repair 2010; 3: 17. |
| [18] | Ries MD, Badalamente M. Arthrofibrosis after total knee arthroplasty Clin Orthop Relat Res 2000; (380): 177-83. |
| [19] | Parvizi J, Tarity TD, Steinbeck MJ, et al. Management of stiffness following total knee arthroplasty J Bone Joint Surg Am 2006; 88(Suppl. 4 ): 175-81. |
| [20] | Zehetgruber H, Grubl A, Goll A, et al. Prevention of heterotopic ossification after THA with indomethacin: analysis of risk factors Z Orthop Ihre Grenzgeb 2005; 143(6 ): 631-7. |
| [21] | Bremen-Kuhne R, Stock D, Franke C. Indomethacin--short-term therapy vs single low dosage radiation for prevention of periarticular ossifications after total hip endoprosthesis Z Orthop Ihre Grenzgeb 1997; 135(5 ): 422-9. |
| [22] | Yercan HS, Sugun TS, Bussiere C, et al. Stiffness after total knee arthroplasty: prevalence, management and outcomes Knee 2006; 13(2 ): 111-7. |
| [23] | Sun Y, De Dobbelaer B, Nackaerts O, et al. Development of a clinically applicable tool for bone density assessment Int J Comput Assist Radiol Surg 2009; 4(2 ): 163-8. |
| [24] | Bosch U. Arthrofibrosis Orthopade 2002; 31(8 ): 785-90. |
| [25] | Zeichen J, Haeder L, Jagodzinski M. Localisation of TGFbeta and PDGF and their relevance for the pathogenesis of arthrofibrosis Unfallchirurg 2008; 111(2 ): 79-84. |
| [26] | Kim J, Nelson CL, Lotke PA. Stiffness after total knee arthroplasty.Prevalence of the complication and outcomes of revision J Bone Joint Surg Am 2004; 86-A(7 ): 1479-84. |
| [27] | Mostafa NZ, Fitzsimmons R, Major PW, et al. Osteogenic differentiation of human mesenchymal stem cells cultured with dexamethasone, vitamin D3, basic fibroblast growth factor and bone morphogenetic protein-2 Connect Tissue Res 2011; 53(2 ): 117-31. |
| [28] | Shore EM. Osteoinductive signals and heterotopic ossification J Bone Miner Res 2011; 26(6 ): 1163-5. |
| [29] | Schauwecker J, Pohlig F, Toepfer A, Gollwitzer H, von Eisenhart- Rothe R. Heterotopic ossifications in total hip arthroplasty: prophylaxis and therapy Orthopade 2011; 40(6 ): 500-. |
| [30] | Leblanc E, Trensz F, Haroun S. BMP9-Induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment J Bone Miner Res 2011; 26(6 ): 1166-77. |
| [31] | Robin BN, Chaput CD, Zeitouni S. Cytokine-mediated inflammatory reaction following posterior cervical decompression and fusion associated with recombinant human bone morphogenetic protein-2: a case study Spine (Phila Pa 1976) 2010; 35(23 ): 1350-4. |
| [32] | Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion Spine J 2010; 10(9 ): 1-6. |
| [33] | Chen NF, Smith ZA, Stiner E, et al. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion J Neurosurg Spine 2010; 12(1 ): 40-6. |
| [34] | Boraiah S, Paul O, Hawkes D, Wickham M, Lorich DG. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report Clin Orthop Relat Res 2009; 467(12 ): 3257-62. |







