Combination of Two Cytokine Inhibitors Reduces Nucleus Pulposus-Induced Nerve Injury More Than Using Each Inhibitor Separately
Abstract
Although recent experimental studies indicate that disc-derived cytokines, as for instance TNF, seems to be intimately involved in the pathophysiology of sciatica and low back pain, the clinical studies performed do not provide conclusive data on TNF-inhibition as a useful complement for treatment of such conditions to existing modalities. Based on the fact that TNF is merely one component in a complex network it was assumed that the combination of a TNF-inhibitor and an IL-1β-inhibitor could potentiate the effects in a pig model on nucleus pulposus-induced nerve conduction velocity reduction. The data indicated that combination of two cytokine inhibitors seems to be more efficient in reducing the nucleus pulposus-induced effects on nerve conduction velocity than using each inhibitor separately. This may be considered if future clinical trials for the treatment of sciatica and low back pain using just a single inhibitor may continue to demonstrate inconclusive data.
INTRODUCTION
There is accumulating experimental evidence that sciatica due to disc herniation and low back pain may relate to activation and sensitization of intraspinal nervous structures by disc-derived substances [1-5]. One key substance for inducing such irritation is Tumor Necrosis Factor alpha (TNF) [1, 6-8]. However, initial clinical trials on TNF-inhibition for treating sciatica have indicated both good [9-12] and less conclusive results [13-16]. Although TNF is considered as a “major player” in inflammatory events, TNF also acts through other pro-inflammatory cytokines such as for instance IL-1, IL-6 and IFN-gamma. One might therefore assume that inhibition of other cytokines in combination with TNF may enhance the effects since one would inhibit the cytokine network at multiple levels. A previous experimental study showed that direct administration of a TNF-antibody at a certain dosage into the nucleus pulposus before application to the cauda equina may only produce a partial reduction of the nucleus pulposus-induced effects on nerve conduction velocity in a pig model [1]. Using this approach, it was assessed if the addition of an antibody towards IL-1 could potentiate this effect.
MATERIAL AND METHODS
Fifteen pigs, (body weight 25-30 kg) received an intramuscular injection of 20 mg/kg body weight of Ketalar® (ketamine 50 mg/ml, Parke-Davis, Morris Plains, New Jersey) and an intravenous injection of 4 mg/kg body weight of Hypnodil® (methomidate chloride 50 mg/ml, AB Leo, Helsingborg, Sweden) and 0.1 mg/kg body weight of Stresnil® (azaperon 2 mg/ml, Janssen Pharmaceutica, Beerse, Belgium). Anesthesia was maintained by additional intravenous injections of 2 mg/kg body weight of Hypnodil® and 0.05 mg/kg body weight of Stresnil®. The pigs also received an intravenous injection of 0.1 mg/kg of StesolidNovum® (Diazepam, Dumex, Helsingborg) after surgery.
Nucleus pulposus was harvested from the 5th lumbar disc through a retroperitoneal approach. Approximately 40 mg of the nucleus pulposus (content of one lumbar disc) was applied to the sacro-coccygeal cauda equina in the same pig through a midline incision and laminectomy of the first coccygeal vertebra. In 5 pigs, the nucleus pulposus was mixed with 100 µg of an anti-TNFα antibody (anti-pig TNFα monoclonal purified antibody, Endogen, Woburn, MA, USA) before application [1]. In five other pigs, the nucleus pulposus was mixed with 100 µg of an anti-IL-1β antibody (anti-pig IL-1β monoclonal purified antibody, Endogen, Woburn, MA, USA), and in the remaining 5 pigs both 100 µg of an anti-TNFα antibody and 100 µg of an anti-IL-1β antibody was mixed with the nucleus pulposus.
Seven days after the application, the pigs were reanaestetized by an intramuscular injection of 20mg/kg body weight of Ketalar® and an intravenous injection of 35mg/kg body weight of Pentothal® (Thiopental sodium, Abbott lab, Chicago, IL). The pigs were ventilated on a respirator. Anesthesia was maintained by an intravenous bolus injection of 100 mg/kg body weight of Chloralose (α)-D(+)-gluco-chloralose, Merck, Darmstadt, Germany) and by a continuous supply of 30 mg/kg/hour of Chloralose. A laminectomy from the 4th sacral to the 3rd coccygeal vertebra was performed. The nerve roots were covered with Spongostane® (Ferrosan, Denmark). Local tissue temperature was continuously monitored and maintained at 37.5-38.0˚C by means of a heating lamp.
The cauda equina was stimulated by two E2 subdermal platinum needle electrodes (Grass Instrument Co., Quincy, MA) which were connected to a Grass SD9 stimulator (Grass Instrument Co., Quincy, MA) and gently placed intermittently on the cauda equina first 10 mm cranial and then 10 mm caudal to the exposed area. To ensure that only impulses from exposed nerve fibers were registered, the nerve root that exited from the spinal canal between the two stimulation sites were cut. An EMG was registered by two subdermal platinum needle electrodes, which were placed into the paraspinal muscles in the tail approximately 10 mm apart. This procedure is reproducible and represents a functional measurement of the motor nerve fibers of the cauda equina nerve roots. The EMG was visualized using a Macintosh IIci computer provided with Superscope software and MacAdios II A/D converter (GW Instruments, Sommerville, MA). The separation distance between the first peaks of the EMG from the two recordings was determined and the separation distance between the two stimulation sites on the cauda equina was measured with calipers. The nerve conduction velocity between the two stimulation sites could thus be calculated from these two measurements.
The person performing the neurophysiologic analyzes was unaware of the experimental protocol for the individual animal. For comparison, data from a previous study of the effects of application of retroperitoneal fat and autologous nucleus pulposus were included [17]. After finishing the complete study the data were arranged in the three experimental groups and statistical differences between each group and the group with nucleus pulposus application without treatment was assessed by ANOVA and Fishers post-hos test.
The local animal research ethics committee approved the experimental protocol for this experiment.
RESULTS
The average nerve conduction velocity for the five groups is displayed in Fig. (1). In a previous study, baseline nerve conduction velocity (fat) was 76 m/s and application of autologous nucleus pulposus induced a reduction of the conduction velocity to 45 m/s [17]. Application of an anti-IL-1β-antibody to the nucleus pulposus did not seem to reduce the effects of the nucleus pulposus (NP-IL-1; p=0,8946). Addition of an anti-TNF antibody was more efficient than the IL-1β-antibody but only produced a partial reduction of the nucleus pulposus effects (NP-TNF; p=0,0788. Most efficient in blocking the nucleus pulposus induced reduction in nerve conduction velocity was the combined action of the anti-TNF and the anti-IL-1β-antibody (NP-IL-1+TNF; p=0,0007). The data from the IL1+TNF group was statistically different to both the TNF group (p=0,0442) and the IL-1 group (p=0,0010).
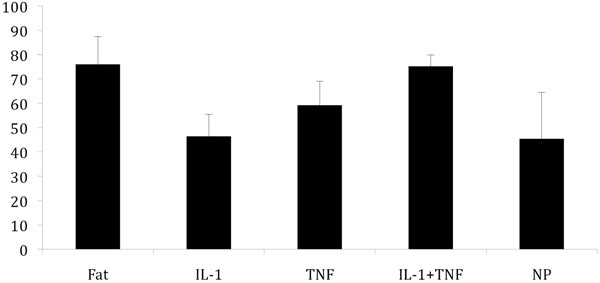
Nerve conduction velocity 7 days after application (±SD) of fat (negative control) [17], nucleus pulposus (positive control) [17] and nucleus pulposus mixed with antibodies towards IL-1β(IL-1) and TNF. Statistically significant difference for these three latter groups compared to nucleus pulposus application was only seen in the IL-1+TNF group.
DISCUSSION
It the present study it was evident that the combination of a TNF-inhibitor and a IL-1β-inhibitor was more efficient in reducing the nucleus pulposus-induced reduction in nerve conduction velocity in a pig model than using each inhibitor separately.
From recent experimental studies is has become increasingly clear that cytokines from the nucleus pulposus may play a role in nerve dysfunction and pain production when the intra spinal nerve tissue is exposed to nucleus pulposus material [1-5]. Most attention has been drawn to TNF but there are also other substances that may be involved in these pathophysiologic events such as for instance IL-1, IL-6 and IFN-gamma [1, 6-8]. Clinical investigations on TNF-inhibition for the reduction of sciatic and low back pain have displayed both good [9-12] and less conclusive [13-16] results. Since TNF is just one substance in a network of synergistic molecules one may suspect that one reason for the absence of clinically detectable efficacy might be overcome if combining inhibition of several inhibitors.
To assess this hypothesis a previously published pig model, in which TNF-inhibition only displayed a partial reduction of the nucleus pulposus-induced effects, was used [1]. In that study a pig anti-TNF antibody was mixed with the autologous nucleus pulposus before application onto the cauda equina. The anti-TNF antibody reduced the nucleus pulposus-induced reduction of nerve conduction velocity from 45 m/s (nucleus pulposus) to 64 m/s, which should be compared to 76 m/s following application of fat tissue used as negative control. In that study, nerve conduction velocity recording were performed 3 days following nucleus pulpous application, but in the present study a 7-day exposure time was selected.
The study showed that also after 7 days there was only a partial reduction of the nucleus pulposus-induced reduction of nerve conduction velocity when an anti-TNF antibody in the same dosage as in the previous publication was mixed with the nucleus pulposus (Fig. 1). Mixing the nucleus pulposus with the same amount of an anti-IL-1β-antibody did not seem to reduce the nucleus pulposus-induced reduction of nerve conduction velocity, whereas combination of both antibodies produced a seemingly complete reduction. The dosages of the two antibodies were chosen without any previous pilot experiments but since they separately only induced incomplete reduction of the nucleus pulposus-induced effects they were regarded to be adequate for the intended purpose.
CONCLUSION
Combination of two cytokine inhibitors seems to be more efficient in reducing the nucleus pulposus-induced effects on nerve conduction velocity than using each inhibitor separately This may be considered if clinical trials for the treatment of sciatica and low back pain using just a single inhibitor may demonstrate inconclusive data.
CONFLICT OF INTEREST
Preliminary data from the present study formed basis for a patent (US 7115557). The corresponding author is part owner of the company Sciaticon AB, Sweden that owns the above-mentioned patent and other patents related to cytokine inhibition for the use of treating spinal pain conditions.
ABBREVIATIONS
| TNF | = Tumor Necrosis Factor (alpha) |
| IL-1 | = Interleukin 1 |
| IL-1β | = Interleukin 1 beta |
| IL-6 | = Interleukin-6 |
| IFN-gamma | = Interferon-gamma |
| EMG | = Electromyogram |
| ANOVA | = Analysis of variance |
ACKNOWLEDGEMENTS
This work was supported by grants from the Swedish Research Council (521-2007-2956), the Gothenburg Medical Society, AFA Insurance, the Gothenburg Medical Society, the Ollie and Elof Ericsson Foundation for Scientific Research, Stiftelsen Olle Engkvist byggmästare, the IngaBritt and Arne Lundbergs Foundation and the Felix Neubergh Foundation.


