All published articles of this journal are available on ScienceDirect.
Use of Carboxymethyl Cellulose and Collagen Carrier with Equine Bone Lyophilisate Suggests Late Onset Bone Regenerative Effect in a Humerus Drill Defect – A Pilot Study in Six Sheep
Abstract
We assessed the use of a filler compound together with the osteoinductive demineralized bone matrix (DBM), Colloss E. The filler was comprised of carboxymethyl-cellulose and collagen type 1. The purpose of the study was to see if the filler compound would enhance the bone formation and distribute the osteoinductive stimulus throughout the bone defect. Six sheep underwent a bilateral humerus drill defect. The drill hole was filled with a compound consisting of 100 mg CMC, 100 mg collagen powder, and 1 ccm autologous full blood in one side, and a combination of this filler compound and 20 mg Colloss E in the other. The animals were divided into three groups of two animals and observed for 8, 12 and 16 weeks. Drill holes was evaluated using quantitative computed tomography (QCT), micro computed tomography (µCT) and histomorphometry. Mean total bone mineral density (BMD) of each implantation site was calculated with both QCT and µCT. Bone volume to total volume (BV/TV) was analyzed using µCT and histomorphometry. Although not statistically significant, results showed increased bone BMD after 16 weeks in µCT data and an increased BV/TV after 16 weeks in both µCT and histology. Correlation between QCT and µCT was R2 = 0.804. Correlation between histomorphometry and µCT BV/TV data was R2 = 0.8935 and with an average overrepresentation of 8.2% in histomorphometry. In conclusion the CMC-Collagen + Colloss E filler seems like a viable osteogenic bone filler mid- to long term. A correlation was found between the analytical methods used in this study.
INTRODUCTION
Autograft is currently the gold standard for enhancing bone repair, although its use is associated with donor site morbidity and limited availability [1, 2]. Besides autograft, banked allograft bone is among the most commonly used osteoconductive and osteoinductive graft materials. Allograft however, holds the risk of infectious disease transmission, and may provoke an adverse immune response despite different processing and preservation methods [3-5]. This has led to numerous osteoinductive bone graft substitutes which vary from single growth factors, such as recombinant human bone morphogenetic protein-2 (rhBMP-2) to compounds containing native growth factors within collagen matrixes derived from demineralized bone matrix (DBM). rhBMP-2 has proven favorable osteoinductive properties, with fast bone regeneration in different orthopedic procedures, but recent studies have shown some undesired side effects when rhBMP-2 is used [6-9]. Safety concerns have been reported, due to formation of edemas, accelerated bone resorption and excessive bone formation [10-13].
Colloss E, a lyophilized complex of extracellular matrix extracted from diaphyseal equine bone, has in recent studies shown to contain mainly collagen type I collagen matrix and other proteins like BMP-2, BMP-3, BMP-7, transforming growth factor-β1 and β2, vascular endothelial growth factor and insulin-like growth factor-1 [14]. It is extracted from healthy homogenous bone tissue and does therefore not vary in quality. The osteoinductive potential from a bovine equivalent (Colloss) was demonstrated in an ectopic rat model, and later Colloss E was proven osteogenic in a porcine spine model [15, 16]. Succeeding studies have shown its effectiveness as an alternative to bone grafts in experimental animal models for long bone repair in sheep, hip implant fixation in dogs, and in an anterior lumbar interbody fusion (ALIF) in a porcine model [17-21]. Furthermore, it has shown improved implant fixation when combined with β-TCP granules in a dog hip model [22].
The bone formation stimulated by Colloss E is known to have similar regenerative metabolic pattern compared to the gold standard graft material, autograft. The bone formation has shown to initiate from the periphery of the defect and inwards, unlike rhBMP-2 where a faster and more widespread ossification is observed [23]. We hypothesize this to be the result of the physical nature of the cotton-like texture of Colloss E, which plasters the matrix to the edges of the bone void. The main aim of this study was to analyze the bone formation when Colloss E was mixed with biocompatible and fast resorbable filler so the osteogenic effect would take effect throughout the bone void. Secondly the aim was to compare some of the most commonly employed tools in current experimental bone reconstructive surgery.
MATERIAL AND METHODS
Colloss E and custom prepared collagen from equine tendons were acquired from Ossacur AG, Oberstenfeld, Germany. Blanose® CMC 7H4XF was bought from Hercules BV, Zwijndrecht, The Netherlands. Colloss E® is a collagenous matrix consisting of Type I collagen chains with various other insoluble proteins including BMP-2 (2.6 mg/g), BMP-7 (3.8 mg/g), TGF-ß1 (55 mg/g) and IGF 1 (2.9 mg/g) [24]. Blanose® consists of Carboxymethyl cellulose (CMC), a cellulose derivative used primarily because of its high viscosity, non-toxicity, and non-inflammatory properties.
The CMC-Collagen powder was packed in sterile vials containing 100 mg CMC and 100 mg Collagen powder. For the control defects, one CMC-Collagen vial was mixed with 1 ccm autologous whole blood giving 1 ccm substance hereafter described as CMC-C. For the intervention defects, one CMC-Collagen vial was mixed with 1 vial Colloss E (20 mg) and thereafter 1 ccm autologous whole blood giving 1 ccm CMC-C with Colloss E.
A total of 6 skeletally mature female landrace sheep underwent treatment under the experimental protocol approved by the Danish National Animal Care and Use Committee. Step-by-step illustration of the surgery procedure is illustrated in Fig. (1). Prior to surgery, the sheep were fasted for two days and kept without water for one day to avoid aspiration during surgery. Under standard aseptic surgical technique and in general anaesthesia with 2% Isoflurane (Abbott Scandinavia AB, Solna, Sweden) and Fentanyl 0.5 ml/hour (Haldid, Janssen-Cilag, Janssen Pharmaceutica, Beerse, Belgium), access to the proximal lateral humeri was made by a transdeltoid approach. On each proximal part of the humerus, the periost was split, elevated and withdrawn from the planned drill hole area. A 2 mm drill guide wire was inserted perpendicular to the cortical surface 8 mm anterodistally to the greater tubercle. With the aid of a wire guide, another two 2 mm guide wires were placed parallel to the drill guide wire, but 8.5 mm proximal and distal to it and later cut at the cortical level. Over the remaining middle 2 mm drill guide wire, a 10 mm deep hole was drilled with a cannulated 11 mm diameter drill bit. After removal of the drill guide wire, the drill hole was irrigated with saline, and the CMC-C with or without Colloss E inserted into the defect. Periost, deltoid fascia, subcutis and subepidermal closure was done with resorbable 3-0 sutures, and skin closure was finalized with metal clips. The animals were kept in the University’s animal housing facility until termination date. Two animals were euthanatized at each time point of 8, 12 and 16 weeks postsurgery, using 1 ml xylazine (Rompun vet.®, Bayer Austria, Vienna, Austria) (20 mg/ml) and pentobarbital (50 mg/kg). The humerus was dissected free, frozen and stored at -20˚C immediately after retrieval.
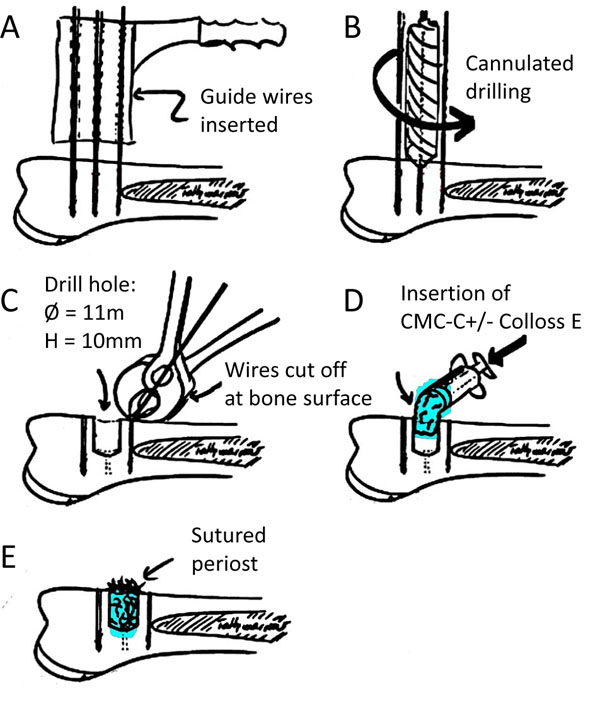
Graphical illustration of operation procedure. (a) Placement of guide wires perpendicular to the one surface and insertion of guide wires. (b) Cannulation of bone defect using cannulated drill bit. (c). Guide wires parallel to the drill hole cut off at the surface of the bone. (d). Insertion of the CMC-C compound with or without Colloss E. (e). Closing of wound with sutures in both periost and skin.
QCT Evaluation
QCT (quantitative computed tomography) was performed on the XCT 2000+ Research scanner (Stratec Medizintechnik GmbH, Pforzheim, Germany). The specimens were oriented in the scanner with the guide wires parallel to the scan plane verified by the scout view scanning. On the scout view image, the center of the drill hole was determined, and three scan planes were sampled: One through the center of the drill hole and one paracentral plane on each side of the central plane, 2 mm from the distal plane. On the three sampled scan planes a 10x10 mm ROI (region of interest) was superimposed on the images guided by the cortical edges and the drill guide wire canal in the bone (Fig. 1). For each of the ROI sizes, a mean total density of the tissue within the three sampled scan planes for each implantation site was calculated.
Specimen Embedding
The drill defect was manually identified using the guidewires parallel to the defect. Using a Proxxon Micro Bandsaw (Greenville, Wisconsin, USA) the region of interest, including the guidewires, was cut out for further embedding. The specimens were dehydrated in a graded series of ethanol (70%-99%) and embedded in methylmethacrylate.
µCT
After embedding, the bone specimens were scanned by high-resolution μCT scanning (µ-CT 40, Scanco Medical AG, Zürich, Switzerland). Cylindrical ROIs of 5 mm and 10 mm in diameter, both with a depth of 8 mm, were chosen. The 5 mm ROI was placed in the center of the drill hole while the 10 mm ROI covered the entire hole. The cylindrical ROIs were found using the guide wires as markers parallel to the drill holes. The scanned images were rendered in three-dimensional (3D) reconstruction and computional algorithms were used to calculate BMD and bone volume fractions (BV/TV).
Histomorphometry
The blocks were cut into sections with a random starting point between 0-800 µm from the top of the drill hole. Five sections were chosen with 1600 µm intervals from the starting point and throughout the length of the drill defect. The thickness of the sections ranged between 40-50 µm. Specimens were colored with toluidine blue, which stains woven bone dark blue and lamellar bone light blue. Intertrabecular space remains uncolored. Blinded quantitative histomorphometry was performed using the stereological software C.A.S.T. Grid (Olympus Denmark AS, Ballerup, Denmark). With the aid of the software, the ROI was defined. In the region, volume fractions of new bone and intertrabecular space were quantified by point counting technique.
Statistics
For correlation comparison between analysis methods, linear regression analysis was used and correlation coefficient (r2) was calculated using STATA software (9.0 software (StataCorp LP, College Station, TX, USA).
RESULTS
No complications were observed during surgery and all animals completed the observation period uneventfully.
µCT
Mineralization parameters measured by µCT show higher BMD and BV/TV values for both animals in the CMC-C + Colloss E group after 16 weeks compared to the CMC-C group. After 16 weeks, a 13% and 15% increase in BV/TV respectively, were observed in the intervention group when compared to the CMC-C control group (Fig. 2). No differences between the intervention and control groups were seen after eight and twelve weeks. When comparing bone formation in the whole defect (Ø = 10mm) with the center part of the defect (Ø = 5mm) for all defects in the CMC-C + Colloss E group, we found BV/TV to be significantly lower in the center part compared to the whole defect by 12 % (p < 0.001). There was no significant difference in center part ROI BV/TV when all control group defects was compared to all defects in the intervention group (p = 0.37) (data on comparison between ROI 5 mm and ROI 10 mm not shown).
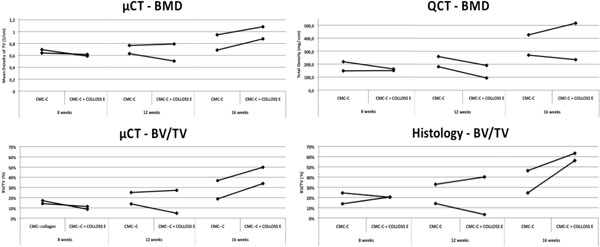
Graphs illustrating the BMD and BV/TV data from QCT, µCT and histomorphometry. The BMD from the µCT data increase after 16 weeks in the Colloss E group, data that cannot be reproduced in the QCT data. The BMD does not vary between 8 and 12 weeks and no difference is observed between the two groups. This is the case for both the µCT and the QCT data. The BV/TV in the bottom two graphs show a similar trend between histomorphometry and µCT. There is no difference in BV/TV between 8 and 12 weeks and no increased BV/TV between control- an intervention group. At 16 weeks both µCT and histomorphometry data show an increased BV/TV in the CMC-C + Colloss E group compared to the CMC-C group.
QCT
Contrary to the µCT results, the QCT data suggested no difference in BMD between the two groups at any time point (Fig. 2), although not significant. Little difference was shown in density between the groups at 8 and 12 weeks. The BMD only increased slightly after an observation period of 16 weeks, which was the case for both groups (Fig. 2). The QCT BMD data correlated with the BMD data from the µCT scans with an R2 = 0.804 (Fig. 3).

Graph illustrating the correlation between the bone mineral density measured by QCT and µCT. The values given by the evaluation software on the machines are given in different units. The Correlation between QCT and µCT data shows strong relation between the two data-sets.
Histomorphometry
Compared to the CMC-C grafted drill defect, the CMC-C + Colloss E treated drill defect showed a higher BV/TV in the 16 week group, which correlates with the µCT data. The increase in BV/TV after 16 weeks was 17 and 32 % respectively, in favor of the CMC-C + Colloss E group (Fig. 2). Correlation between the histological observations and µCT (Fig. 4) were found to be strong with a correlation coefficient, R2 of 0.8935. The average percentage difference was found to be 8.2% between BV/TV measured with µCT and histomorphometry, with histomorphometry resulting in the highest values. New bone formation was primarily located in the circumference of the bone defect. New bone seemed to be formed from the walls of the drill hole and inwards and a multilocular reossification could not be observed. In most of the drill holes, the inner part was still only marrow space as illustrated in the comparative illustration between histological sections and µCT 3d reconstructions in Fig. (5). After 8 weeks, bone formation was primarily appositional on the defect walls in the CMC-C group. In the Colloss E group, intramembranous bone formation was the major mechanism observed but strictly situated to the periphery of the defect (Fig. 5A, B). After 12 weeks, new woven bone was visible almost throughout the defect and lamellar bone was being formed by remodeling close to the edges of the defects in the CMC-C + Colloss E group. In the CMC-C group, no or little lamellar bone was present and woven bone was not present in nearly the same amounts as in the intervention group (Fig. 5C, D). The same morphologies were present after 16 weeks but the discrepancy in lamellar bone were more distinct at this time-point between the groups favoring the CMC-C + Colloss E filled defects. Furthermore, newly formed trabecular bone was present in the periphery of the intervention group defects (Fig. 5E, F).
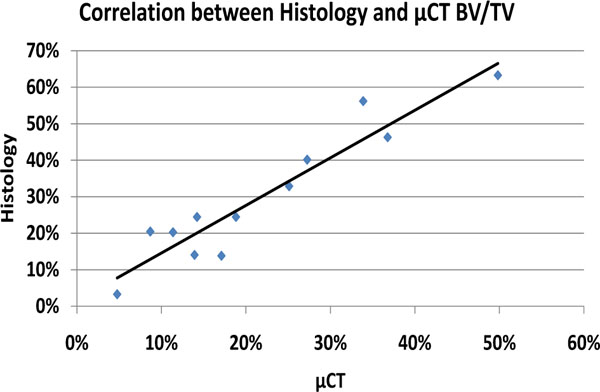
Graph illustrating correlation between BV/TV from µCT and histomorphometry. The correlation between the data was satisfactory although with a systematic overrepresentation of Bone Volume in the histomorphometry data.
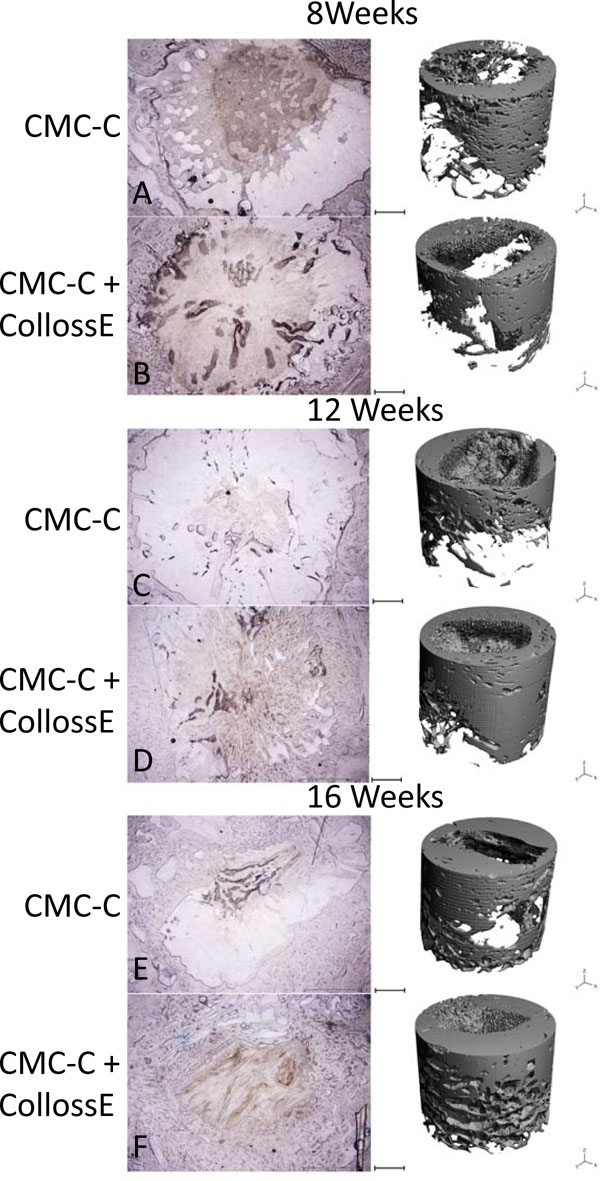
Comparison of representative histological slices with 3-dimensional reconstructions of the drill defects for control and intervention group at all three time points. In the histological, the bone is colored blue with toluidine blue staining. The images show approximately 80% of the drill hole. The drill hole is easily recognizable with its uncolored marrow space surrounded with blue colored bone. In the CMC-C group, no or little bone formation is present (A), whereas peripheral membranous bone formation is visible in the CMC-C + Colloss E group (B). After 12 weeks, lamellar bone formation was observed at the edges of the defect in the intervention group and woven bone was present throughout the outer parts of the defect (D). No lamellar bone was observed in the control group (C). After 16 weeks, lamellar bone in newly formed trabecular bone structures was visible in the periphery of the intervention group bone defect and new bone was observed almost throughout the defect (F). The trabecular formation was less extensive in the control defect (E). There was no multilocular reossification present at any time point. The 3D reconstruction supports these observations on the right side of the illustration. Bars between the two columns represent 2 mm on the µCT reconstructions and 1,2 mm on the histological images.
DISCUSSION
This study revealed a late-onset bone regenerative effect of Colloss E. The bone healing was the same for both the intervention and the control group until after 16 weeks where the results suggests increased bone healing in the intervention group. The osteoinductive effect of Colloss E has been validated in previous studies and with earlier onset of the bone regenerative effect, which would indicate a delayed response from the osteogenic compounds known to be present in the lyophilizate. This could be due to an encapsulation of the osteoinductive components by the CMC-C. Many osteogenic compounds are using carriers to slow the release or act as fillers [21, 25]. One of the most widely used is collagen type-1, which is primarily known for its use as a carrier for commercially available BMP-2 (INFUSE, Medtronic Sofamor Danek, Memphis, TN, USA) [26, 27]. Previous studies however do not suggest such an effect from the carriers selected. The mixture of collagen type-1 and CMC in weight ratio 2:1 has previously proven to be a favorable carrier for osteogenic protein-1 (rhOP-1) [28]. The choice of a mixture between CMC and Collagen type-1 in weight ration 1:1 was based on these previous findings as well as the favorable viscous properties of CMC when added to a liquid such as whole blood. This mixture was added to increase the volume of the compound, and thereby distribute the bioactive lyophilisate evenly in the defect, minimize spillover to adjacent tissue and improve handling. These qualities were met with the exception of bone formation activity throughout the drill defect. By adding CMC-C to Colloss E, we expected the osteoinductive effect to increase in the center of the drill defect. By applying a more regular distribution of Colloss E within the bone defect during the biodegradation of the carrier, we expected small patches of carrier and Colloss E to be trapped within the newly formed bone and thereby facilitate a centripetal ossification with developing multilocular ossification sites progressing from the periphery of the drill defect towards the center. The results did however not cause any increased bone formation in the central part of the defect with a non-significant difference between control and intervention group BV/TV in the 5 mm center ROI. This leads to the suggestion that the CMC-C carrier is unable to facilitate multilocular reossification within the defect as seen when osteoconductive material or high dose BMP-2 is added to a bone defect [21]. A recent study have been investigating the addition of an osteoconductive component to Colloss E. Nienhuijs et al. used β-TCP granules in addition to Colloss E in a goat mandible defect and although bone formation was observed around the β-TCP granules in the central part of the defect, significantly less bone was present when compared to an empty defect and a defect with Colloss E alone [29]. The lack of new bone formation was explained by a high degree of inflammation partly due to foreign body reactions and excessive growth factor stimulation caused by the Colloss E. The use of CMC-C could potentially minimize this undesired effect by acting as a slowly degrading reservoir for the osteoinductive factors in the Colloss E matrix, minimize foreign body reaction and swelling, and thereby enable the use of an osteoconductive component in conjunction with Colloss E. The amount of Colloss E used in this study is important to take into consideration. Previous studies have used double the amount of Colloss E in the same size bone defect [19, 21]. The concentration of growth factor may thereby be in sub effectual quantities for rapid onset of bone formation and early osteogenic response and the insufficient short-term effect further enhanced by retention of growth factor within the filler material. Collagen is also known to have a role in the differentiation of immature mesenchymal stem cells into osteoblasts in itself, which could result in a positive osteogenic effect in the control group and thereby masking the effect of Colloss E [30].
The histological methods are considered gold standard for measuring bone volume to total volume. However, many other analytical methods are available to validate the histological findings as well as providing additional data regarding three dimensional bone structure and trabecular morphology. It is, however important to notice the limitations of these methods when correlating to traditional histological data. µCT is known to underestimate bone volumes when small quantities of bone are found. Furthermore µCT overestimates bone volume when large quantities are present [31]. In this study we found strong correlation between BV/TV measurements from µCT and histomorphometry (R2 = 0.8935). However, we found an average difference of 8,2% with histomorphometry analysis resulting in a higher BV/TV. This phenomenon could be due to section thickness bias. With histological sections ranging in thickness from 40 to 50 µm the chance of systematic overestimation is present. Tissue over projection occurs at the interface between two tissues of different opacity, where the more opaque tissue will be over projected. This will cause a systematic overestimation of the volume fraction of the more opaque tissue. This phenomenon is directly related to section thickness and inversely related to size structure [32]. This bias could be avoided in future studies by choosing thinner sections.
The limitations of the study are mainly caused by the limited amount of animals in each group. This excludes statistically significant results from the differences in bone volumes and mineral densities to be concluded. Furthermore the lack of a positive control, such as autograft, leaves an important comparison unanswered. Instead this study sheds light on future approaches, which could lead to a more homogenous and prolonged bone regenerative effect when DBMs are used to heal bone defects.
CONCLUSION
This study evaluated the possibility to mix the osteoinductive bone matrix lyophilisate, Colloss E, with a high viscosity filler material, The resuslts suggests an increased osteogenic effect of the CMC-C + Colloss E after 16 weeks. There was no increased bone formation in the center of the defect in the CMC-C Colloss E groups when compared to the CMC-C group. The bone quantity methods used in the study correlated with each other and proved consistent in showing equivalent results.
DISCLOSURE
Unconditional supply of COLLOSS E and Collagen by Ossacur AG, Oberstenfeld, Germany.


