RESEARCH ARTICLE
Basic Science Considerations in Primary Total Hip Replacement Arthroplasty
Saqeb B Mirza*, 1, Douglas G Dunlop2, Sukhmeet S Panesar3, Syed G Naqvi4, Shafat Gangoo5, Saif Salih6
Article Information
Identifiers and Pagination:
Year: 2010Volume: 4
First Page: 169
Last Page: 180
Publisher ID: TOORTHJ-4-169
DOI: 10.2174/1874325001004010169
Article History:
Received Date: 20/10/2009Revision Received Date: 10/11/2009
Acceptance Date: 5/3/2010
Electronic publication date: 11/5/2010
Collection year: 2010

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Total Hip Replacement is one of the most common operations performed in the developed world today. An increasingly ageing population means that the numbers of people undergoing this operation is set to rise. There are a numerous number of prosthesis on the market and it is often difficult to choose between them. It is therefore necessary to have a good understanding of the basic scientific principles in Total Hip Replacement and the evidence base underpinning them. This paper reviews the relevant anatomical and biomechanical principles in THA. It goes on to elaborate on the structural properties of materials used in modern implants and looks at the evidence base for different types of fixation including cemented and uncemented components. Modern bearing surfaces are discussed in addition to the scientific basis of various surface engineering modifications in THA prostheses. The basic science considerations in component alignment and abductor tension are also discussed. A brief discussion on modular and custom designs of THR is also included. This article reviews basic science concepts and the rationale underpinning the use of the femoral and acetabular component in total hip replacement.
1. INTRODUCTION
THR is one of the most common operations performed on the NHS. About 40000 primary THRs are performed in NHS hospitals in England with about 4000 revision procedures being performed [1]. An increasingly ageing population means that absolute numbers of people with a predilection for osteoarthritis is set to rise. It is estimated that THRs will increase by 40% over the next 30 years due to demographic change [2] and it is projected that the highest rate of increase will be in the middle aged and over 85s. There are differing guidelines in terms of indications for THR among different a countries, which makes direct comparison of data difficult to make. Although being a very cost-effective operation (THR cost-utility analysis estimates that the cost per QALY is £700, compared to £3000 for a kidney transplant), the cost to the NHS is still substantial and in 1992 was estimated at 231.3 million pounds [3].
There are numerous types of femoral components made by different companies and many have been introduced into the market within the last 5-10 years and hence long-term follow-up data is not available on a large proportion of them.
This article aims to provide a basic scientific understanding of the rationale behind the use of different varieties of acetabular and femoral component for THR used in different situations. It is by no means intended to provide hard and fast rules on their use.
2. RELEVANT JOINT ANATOMY IN THA
The hip is a ball and socket joint in which stability is obtained by the bony configuration combined with a complex system of muscles and ligaments around the joint. The femoral head diameter averages about 46mm. Two critical angular relationships of the femoral neck with the shaft [4] include the neck shaft angle which averages 130 degrees and the femoral anteversion angle which averages 12 degrees. Femoral neck version is the angle of the femoral neck with the intercondylar plane. The hip joint contribution to lower limb length is the vertical distance from the femoral head centre to the lesser trochanter. Femoral offset is the horizontal distance from the midline of the longitudinal axis of the femur and the centre of rotation of the femoral head (Fig. 1). Individual variations and conditions that affect head neck angle and femoral anteversion lead to changes in femoral offset and hip joint contribution to limb length [5]. For example patients with hip dyplasia may have coxa valga and increased femoral anteversion, with resultant decrease in offset and increase in limb length while in coxa vara, the femoral neck angle is reduced, leading to greater offset and tendency to shortening. These conditions also pose a challenge in THA and need careful preoperative consideration. Femoral head diameter is normally at least 1.2 times the neck diameter. Anterior impingement may result with lesser ratios [5]. Acetabular anteversion is the amount of forward flexion of the acetabulum as measured from lateral to medial with reference to the sagittal plane and averages about 15 degrees. The acetabular abduction angle is the relationship of the line extending from the anteromedial and superolateral extents of the acetabulum with the horizontal. The acetabulum averages 15 degrees of anteversion and 45 degrees of abduction (Fig. 2). The natural curve of the femur in an antero posterior direction is about 4 degrees. It is also important to note the three types of femoral shape based on metaphyseal-diaphyseal anatomy. Dorr type A femurs have wide metaphyses and narrow diaphyses, type B have a smooth metaphyseal-diaphyseal transition and type C do not have much difference in the sizes of these two regions.
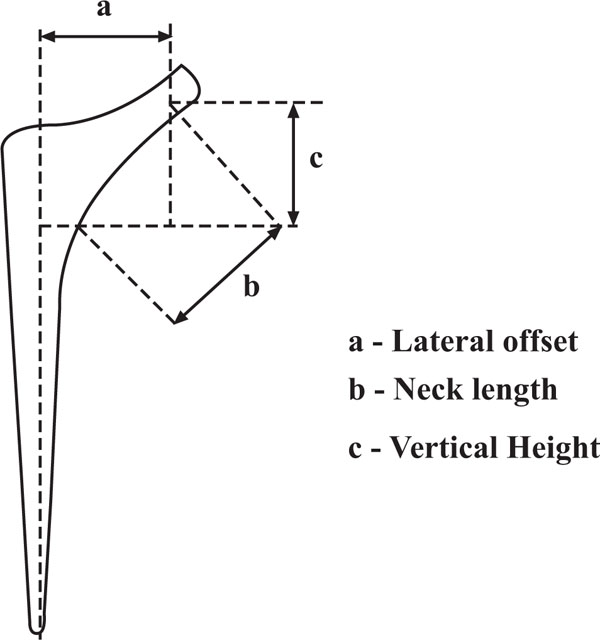 |
Fig. (1). Schematic diagram showing parts of a standard femoral component for THA. |
 |
Fig. (2). Schematic diagram showing acetabular coronal tilt and anteversion angles. |
3. BIOMECHANICAL CONSIDERATIONS
Kinematically, the three axes of hip joint movement are flexion-extension, abduction-adduction and internal-external rotation. Finite element modelling (FEA), a computer-generated method of analysing stresses across an artificial joint surface or predicted stresses across a material [6, 7] can be used to simulate changes across the artificial joint as part of surgical planning. It is estimated that the hip joint has to withstand each year, with cyclical loading, an equivalent of 3-6 times body weight due to contraction of the abductors [8], and peak loading 106 steps of 7-8 times body weight is seen in sporting activities.
The system can be simplified to consist of a lever arm where the hip joint is the pivot and forces on the femoral head are equal and opposite to those on the acetabulum. An upward force is generated at an angle by the resultant of the abductors, and the body weight generates a force that acts vertically downward (Fig.3). The ratio of the body-weight lever arm to the abductor lever arm is approximately 2.5:1.0. The abductors provide two thirds of the hip joint force [9] parallel to the long axis of the femur [10, 11]. The resultant force across the hip joint in the frontal plane makes an angle of 15-25 degrees to the long axis of the femur, producing axial compression, a varus moment and a medial-to-lateral force [12]. It can be seen that with increasing offset and/or cup medialization that resulting joint reaction force (JRF) can be reduced, which is one of the surgical principles of THA advocated by Sir John Charnley. In the sagittal plane, a torsion force is created by the anteroposterior component of the resultant forces [13, 14]. This is equivalent to an axial torque on the femoral component similar to a wheel brace used to tighten nuts on a car wheel. FEA also shows high stresses at the bone-implant interfaces [15], maximum in the proximo-medial and the distal-lateral regions, though their magnitudes may vary with particular prosthetic designs and materials. This explains why these areas are often preferentially worn in loose femoral components, seen at the time of surgery as excessive wear. Local contact stresses as well as shear stresses play a role in uncemented versions of prosthesis [12].
 |
Fig. (3). Schematic diagram showing the Joint Reaction Force generated by the abductor lever arm. |
4. MATERIAL AND STRUCTURAL PROPERTIES
Young’s modulus of a material is defined as stress divided by strain where stress is given by force per unit area and strain by change in length as a function of original length. It is a property not governed by shape as it has no units, but given similar shaped items, defines the different sensations of flexibility when made of materials with different young’s moduli. In artificial materials the elastic modulus is often linear, while biological materials including bone usually display viscoelasticity, whereby it’s material properties are time-dependant and depend on the rate of loading. Viscoelastic materials often display hysteresis where loading and unloading curves do not exactly overlap, and energy is lost within the material as internal friction.
Bone is an anisotropic material. This means that it’s mechanical properties are greater in one direction than another, due to alignment of collagen fibrils and osteons [16]. Bone also exhibits creep, whereby when subjected to a constant load for an extended period of time, will continue to deform at decreasing rates. The converse is true when subjected to a constant deformation rate and is termed stress relaxation. Strain rate is also important, whereby rapid application of even a modest force can lead to fracture, compared to a higher force at a lower rate. Bone and implants used for THR have different material and structural properties and together, form composite beam structures that function together.
5. BASIC TYPES OF TOTAL HIP ARTHROPLASTY
Types of arthroplasty are commonly described with reference to modes of fixation into cemented and uncemented types.
5.1. Cemented Components
Polymethylmetacrylate (PMMA) is the material commonly used for prosthetic stabilisation. This is based on the concept that cement interdigitates within bone and that PMMA is stronger in compression than in tension [17]. Cement acts as grout and therefore there is no true adhesive bond between the prosthesis and bone. During initial cementing, mechanical, vascular, thermal and chemical trauma play a role in disturbing normal bone function. The endosteal blood supply is damaged and endosteal necrosis occurs up to a depth of 500 micrometres. Over the ensuing months this blood supply is re-established as fibrovascular granulation tissue and a new interface between cement and bone is generated [18].
5.2. Uncemented Components
This is thought to represent a truly biologic method of implantation in that the coated surface of the metal implant encourages in-growth or on-growth of bone onto the implant. Cortical bone grows into the porous channels within the metal implant to create a rigid interface. The philosophy behind uncemented THR is the establishment and maintenance of a rigid bone-implant interface that has remodelling potential such that bony intercalation into the implant can be re-established in areas of disruption [19]. Uncemented components may be surface-engineered in two ways to encourage bony interlock. Porous coating is where the implant surface has been treated to have many microscopic pores of varying depth, into which bone may grow. Grit blasting bombards the implant with microscopic particles that create indentations on the implant surface onto which bone can grow.
6. BEARING SURFACES
Traditionally, two types of bearing surfaces have been utilised in THA. Hard-on-soft bearings have included couplings where the acetabular liner has been polyethylene (PE) and the femoral heads have been metal, usually cobalt-chrome, or ceramic. Recent advances in these articulations have included processes to improve surface hardness and resistance to adhesive and abrasive wear of the PE component and to reduce the rate of aseptic loosening and osteolysis. Hard-on-hard bearing surfaces have included ceramic-on-ceramic (COC) or metal-on-metal (MOM) surfaces and these have gained popularity because of significantly lower wear rates than hard-on-soft bearings.
7. MECHANISMS OF WEAR IN THA BEARINGS
Three main types of wear have been recognized in THA couplings. Abrasive wear is caused by two surfaces of different hardness articulating against each other causing particles to be removed from the less abrasive substance with less surface hardness. Adhesive wear is most commonly caused in PE articulations where PE particles are sheared off and deposited within the joint space, stimulating an osteolytic reaction. Third-body wear is when particles entrapped between two contacting and articulating surfaces causes wear of the softer articulating surface.
8. ASEPTIC LOOSENING AND OSTEOLYSIS
It is currently thought that wear particles generated from the acetabular polyethylene are the main inducers of the macrophage and histiocytic response that leads to osteolysis [20]. Aseptic loosening is thought to be initiated by a combination of mechanical factors including cyclical loading and impingement of the neck on the acetabular margin. Gruen [21] described four mechanisms of cemented component failure. The first is pistoning where the stem and/or cement subside into the femur. The second is the medial midstem pivot where a varus positioned stem fails at the proximo-medial and distolateral areas. Calcar pivoting is the third where the distal aspect of the stem can shift within the distal cement mantle. Loss of cement mantle proximally and fatigue failure of the proximal stem due to repetitive loading at a compromised cement-implant interface is termed cantilever bending. Loosening has been demonstrated even before the fibrous membrane forms and is possible evidence that the initiating factors are mechanical [22]. Biological factors then cause further loosening, leading to progressive osteolysis, undermining the remaining bony support, which when severe, can lead to macroscopic rather than microscopic loosening, with subsidence and risk of periprosthetic fracture. In the articulation, adhesive and abrasive wear generates wear particles from the softer material, typically PE. Biologically active wear particles range from 0.1-10 micrometres with those in the range of 0.1-0.5 micrometres being the most potent. Phagocytosis of these particles by macrophages cause release of cytokines and prostaglandins into bone stimulating osteoclastic activity and causing osteolysis [23]. Release of oxide free radicals and hydrogen peroxide also cause bone resorption. Inflammatory mediators including interleukin-1 (IL-1), interleukin-6 (IL-6), tumour necrosis factor alpha (TNF-α), prostaglandin-E2 (PGE2) and tumour necrosis factor-beta (TNF-β) stimulate osteoclasts. Monocyte colony stimulating factors (M-CSF) and granulocyte colony stimulating factors (G-CSF) stimulate osteoclast precursors. Interleukin-1 also inhibits osteoblast function. Other mediators such as collagenase and metalloproteinases cause direct bone osteolysis [23, 24]. Huge numbers of wear particles have been demonstrated from prosthetic joints [25] with an estimated 38000 particles per step for a 22mm prosthetic head [24]. Although several studies have suggested a critical wear volume related to the occurrence of osteolysis, other factors that have been shown to influence the extent and severity of the osteolytic reaction include total number of particles, size and morphology of particles with irregular shapes being more immunologically active than spheres [23, 24].
9. CONCEPT OF EFFECTIVE JOINT SPACE (EJS)
This concept describes the entire volumetric area within the hip arthroplasty construct, that can theoretically be infiltrated with wear particles and macrophages, potentially causing osteolysis [19]. For example, the use of bone screws for the fixation of metal shells increases the effective joint space. An understanding of this concept is important as any reduction in the EJS could, in theory, reduce the area that can potentially undergo osteolysis.
10. DISCUSSION
10.1. Cement Fixation
Third generation cementing techniques that include vacuum preparation of cement to reduce porosity and increase cement strength, distal plugging of the canal [26,27] are now commonplace and we certainly use them in our practice even though the evidence for some of them may not be fool proof [28]. Preparation of the femoral canal by pulsatile lavage and brushing and the use of a cement gun and cement restrictors significantly improves the quality of cement-bone interdigitation [29]. We also use sponges impregnated with adrenaline or hydrogen peroxide to ensure adequate haemostasis at the bone-cement interface just prior to cementing. Using cement centralizers both proximally and distally where applicable ensures a uniform cement mantle of 2-4mm [30, 31] to prevent loosening and fractures in the cement mantle in regions of increased metal-bone proximity. The size of the cement mantle is controversial [28]. A cement mantle of less than 2mm has been shown to demonstrate increased cracking and breakdown [30, 32, 33] and therefore our opinion is that it is best to maintain a greater than 2mm cement mantle circumferentially, but particularly in the proximomedial part of the femur. In our practice we try and establish a 4mm cement mantle in this region as this is an area of increased stresses. Avoiding gaps in the cement mantle is of paramount importance to prevent areas of direct contact between the prosthesis and bone that lead to stress risors and early failure [34, 35] and to prevent localized endosteal osteolysis [36]. Varus stem positioning results in a thin cement mantle in the proximal medial and distal lateral regions of the femur where the cement mantle experiences the highest stress levels [33, 37] and can lead to cracking and failure. There is a variable relationship between the medial aspect of the greater trochanter and the centre of the femoral shaft and errors in positioning of the femoral component may result secondary to an inappropriate entry point. The piriform fossa serves as a reliable entry point for femoral preparation as its position remains relatively constant. Thus we use this point as our starting point for femoral preparation in many of our THAs.
A reduction in longitudinal compressive stresses has been demonstrated especially proximomedially by about 20-30% [38, 39], particularly when stems with a high elastic modulus are used, causing stress shielding [40]. A mismatch between relative stiffness of the implant and the host skeleton determines the severity of stress shielding. Materials with higher elastic moduli cause more stress shielding than those with lower ones, as do larger diameter stems and implants placed more eccentrically than centrally [18]. Osteopaenia and resorption of bone away from cement over many years may increase interface stresses [41] thereby contributing to loosening [21]. Many stems are currently made from titanium alloy to exploit the beneficial effect of having an elastic modulus closer to that of bone and hence lower bending stiffness. This transmits load to the proximo-medial bone more efficiently to avoid stress shielding.
Smooth contours and sectional shapes that do not twist within the cement mantle are usually utilized to increase rotational stability. Sharp edges on implants are avoided as they are a cause of stress risors within cement [42, 43]. Stems with rounded medial borders reduce stress concentration at the medial cement mantle where failure can occur [44]. Proximo-lateral projections increase the connection between the stem and cement, increase compressive stresses and reduce tensile stresses within this region. Stem designs that are broader laterally than medially diffuse compressive stresses medially and increase torsional and bending rigidity [42].
A femoral stem functions either as a composite beam or a taper slip model with both having different mechanisms of load transfer. In the composite beam model, the stem is considered a rod within two tubes, cement and bone, and depends on strong bonding between both interfaces to form a stable construct from three materials with different mechanical properties (metal, cement and bone). Load is transmitted via the femoral head and stem to it’s tip, bypassing the proximal femur and thereon to the bone cement and subsequently to host bone [45]. A loaded, polished taper stem on the other hand, must be able to move within it’s cement mantle to function as a loaded taper. The load is transmitted from the prosthetic head and forces the taper to subside within the cement mantle, creating radial compressive forces within cement and hoop stresses within bone, thus minimizing proximal stress shielding [46]. Thus these two biomechanical systems require different prosthesis-cement interfaces, a perfect stem-cement bond for the composite beam system but no bond between the stem and cement in the taper slip design [47].
For our cemented implants, we generally use smooth polished stems that allow physiologic subsidence due to viscoelastic properties [48] of cement during cyclical loading. The quality of the cement mantle in Gruen zone 7 ensures most of the load is transmitted to the proximal one third of the femur [21]. Rough surfaces on cemented stems have not been successful due to increase in shear and tensile stresses at the interface, causing progressive debonding and interface micromotion [41, 49]. Cement fixation does create two separate interfaces. These include the cement-bone and the cement-implant interface, with no remodelling potential at either one of them. In cemented prosthesis, a strong adjacent cortex is required for success to provide sufficient cement interdigitation for long term fixation [20]. Mechanical lock is central for cement fixation in any situation, and this becomes a particular problem where large cavitory defects exist and a smooth bony surface usually prevents cement interdigitation. The success rate of cemented revision THRs has thus been poor [50-52]. Indeed Katz demonstrated a 26% failure rate with the use of cemented revision prosthesis [53]. Another study looking at the shear stresses at the bone-cement interface demonstrated this figure in the femur to be only 20.6% of primary strength after a single cemented revision and only 6-8% after a second cemented revision [54]. In Dorr type C femurs or in patients who have poor bone quality in whom uncemented components are not likely to do well, we use cemented implants. PMMA-coated femoral implants have been shown to improve the interface between the stem and cement mantle, but have not resulted in lower rates of loosening [55, 56]. Overall, cemented components have demonstrated excellent long term survivorship with revision rates of 0-5% at more than10 year follow-up [57, 58]. In our experience and according to the literature however, cemented acetabular components are not routinely suitable for younger more active patients but should be used in the older, lower demand patients and patients with soft bone, for example rheumatoids as well as in those with acetabular protrusio [59]. Failure rates of 10-23% at 10 years have been reported [37, 55, 58]. The quality of the cementing technique is very important in acetabular cup loosening [60]. Causes of failure include poor operative technique [61, 62], failure to remove all articular cartilage at the periphery or poor pressurization [62]. In our practice we routinely use flanged sockets to establish the best bone-cement interface at the time of surgery. Indeed, these have been shown to be effective with higher peak pressures and higher intruded cement volumes being obtained [62-64]. Poor acetabular bone quality on average reduces the longevity of the cup and therefore the acetabulum must be reconstructed with grafts or metallic devices when necessary before cementing in the cup, to ensure sufficient fixation [65].
Despite problems with cemented implants, long-term results do demonstrate that cement fixation does provide stable long-term fixation. Attention to detail of the technical aspects of cement fixation and a good understanding of the basic science principles underpinning this method are paramount for success and we continue to use it extensively in our practice.
10.2. Uncemented Components
Many studies have demonstrated increased failure rates of cemented components in younger more active patients. Other studies have demonstrated improved performance and decreased loosening in patients who have had uncemented components in the short to medium term [66]. This, combined with the theoretical advantage of having a potential life-long bond between the implant and host bone due to remodelling potential at the interface, has led to an increase in uncemented THAs.
10.2.1. Material Considerations
Cobalt-chrome alloys are extensively used for manufacture of implants. They have a high ultimate strength, are biocompatible, easily workable and relatively resistant to corrosion. However, they do have a high modulus of elasticity and thus a higher bending stiffness and could contribute to stress shielding. Long-term effects of metallosis arising from cobalt-chrome are unknown and is the subject of research in many labs.
Stainless steel is still used for the manufacture of some femoral implants despite it’s tendency to corrosion and lower fatigue strength. Some of the most successful implants are made from stainless steel due to it’s relatively high strength, cost and easy workability.
Titanium-alloy has been increasingly used for femoral stem manufacture due to it’s superior biocompatibility and relatively lower elastic modulus, thus reducing the problem of stress-shielding. It is corrosion resistant due to the formation of a protective titanium-oxide layer on the surface by spontaneous passivation. It’s surface is also easily modified to enhance osseointegration [67]. However, it is softer than other metals and cannot be used for femoral heads due to wear. Its is also easily scratched and it’s notch-sensitivity may reduce fatigue life of the implant [18, 24].
10.2.2. Surface Engineering and Modification
Establishment and maintenance of a durable connection between the implant and host skeleton underpins the success of cementless fixation. Success is ensured by close contact between the implant surface and host bone, minimizing relative motion at the interface and appropriate surface characteristics [18]. Surgical trauma during preparation of the bony bed for the implant and placing the implant into the canal is thought to stimulate mesenchymal stem cells on the endosteal surface to become osteoblasts, subsequently start laying down extracellular matrix which eventually becomes mineralized via the intramembranous pathway [18]. Implant stability at the time of surgery and intimate contact between the implant surface and viable host bone are critical to success. It is important to keep the distance between the implant and host bone to a minimum of 50μm to enable osteogenic cells the bridge the bone-implant interface. Although interface gaps of up to 1mm can be bridged, there is a risk of excessive interface motion. Excessive interface motion of more than 30-150μm leads to fibrous tissue formation rather than a rigid bony interface [19, 68]. Achieving rigid fixation with the line-to-line reaming technique may sometimes be challenging and the use of supplementary fixation with screws may be required to maintain rigid fixation. Screws however, may increase the effective joint space, decrease the acetabular surface area for bony in-growth and potentially increase the extent of osteolysis. In addition, screw backout onto the backside of the PE insert has been demonstrated to cause backside wear [69]. Our preference is not to use screws if a reasonable fix is achieved as studies have demonstrated a 99% 12 year survival of acetabular shells without screw fixation [19]. Recently, better designed locking mechanisms between the shell and the liner have replaced previous designs which allowed motion between the PE liner and the shell, contributing to backside wear [70]. Press-fit systems where the bone is reamed to 1-2mm smaller than the actual component, depend on hoop stresses to achieve primary stability and do not require supplemental fixation. However, one needs to be careful about the risk of fracture. In our experience, under-reaming by 3-4 mm significantly increases the risk of intraoperative fractures. Detection of an intraoperative fracture is a good indication for acetabular screws [71]. Trabecular metal is a new type of material made of tantalum used for porous monobloc acetabular implants with an integrated PE liner, potentially eliminating the risk of backside wear [72, 73] and has shown promising bone ingrowth and mechanical fixation and, in an experimental model, superior bony gap healing and less migration of PE particles in peri-implant tissue [74]. However, long-term results are lacking.
Studies have demonstrated that in porous coated implants, pore sizes that optimize bony ingrowth range between 100-400μm [75-77] and optimum pore density is 40-50% [75], beyond which the porous coating may actually be sheared off the implant. It is thought that in grit-blasted prosthesis, a large proportion of the surface needs to be covered, and the higher the surface roughness, the higher the risk of abrading cortical bone, producing metal debris [20].
The extent of porous coating in primary hip arthroplasty is controversial. We generally use implants that have been circumferentially porous coated to encourage bony ingrowth from all directions and to avoid development of stress risors at points between coated and uncoated sections in non-circumferentially coated stems. Non-circumferentially coated stems fail to establish bony ingrowth around the entire perimeter of the proximal femur and allow access of PE debris to the femoral diaphysis. In effect, the effective joint space is increased causing femoral diaphyseal osteolysis [78]. Using circumferentially coated stems allow bony ingrowth around the entire circumference of the stem, thereby sealing off the effective joint space from the femoral diaphysis. Indeed, circumferentially coated stems have performed better than their non-circumferentially coated counterparts with regards to osteolysis [78, 79]. In our practice we tend to use femoral stems that are proximally circumferentially coated for our primary THAs. Proximally coated femoral stems achieve fixation in the proximal metaphyseal region, thereby transmitting forces in a more physiological fashion to avoid stress shielding. Fully coated implants achieve fixation throughout the length of the implant due to the large surface area of coating. Substantial amounts of force loads are thus transmitted to the more distal aspects of the femur at the expense of the proximal parts, causing stress shielding and proximal bone loss. In addition, they may cause increased thigh pain. One FEA study demonstrated that coating should be present on the upper 50% of the implant to avoid stress shielding [80]. It has been shown that proximal bone loss from stress-unloading of the proximal femur using extensively porous coated implants is progressive, albeit stabilizing after two years [81]. However, in a revision situation, extensive porous coating is beneficial as distal fixation is to be relied upon for implant stability [53] and prostheses which have limited proximal coating have significant failure rates in this situation as this relies on maximum bone contact in the metaphysic where bone stock is often deficient [82].
Survival rates as high as 98.8% at 10-year follow-up have been demonstrated for uncemented coated acetabular cups [66] and we continue to use them in our higher demand younger patients. Similarly, for uncemented femoral components, loosening rates of now less than 0.5% annually can be achieved [83]. Intraoperative fractures are more common in femoral uncemented components [84] and one must be careful especially in the calcar region when inserting them.
10.3. Bearing Surfaces
Hard-on-soft bearings have included metal on PE articulations and more recently ceramic on PE. The metal on PE bearing has been the most common bearing surface and has proved to be economical due to it’s low cost and ease of manufacture combined with good long term results especially in lower demand individuals. Titanium heads have been least favourable due to high rates of volumetric wear, notch sensitivity and third body wear [20, 85] and is no longer used for head manufacture. Cobalt-chrome on PE bearings have had reasonable long-term success. The problems of degradation of mechanical properties of PE and increased wear rates [86, 87] due to previous gamma irradiation in air and oxidation on the shelf [70, 88, 89] have now partially been overcome by enhanced cross linking and stabilization procedures that include exposing it to a sequence of radiation in an inert environment combined with an annealing or melting procedure [90]. PE now has excellent wear properties due to processing to achieve enhanced crosslinks producing ultra-high molecular weight PE (UHMWPE). This has led to improved surface hardness and enhanced resistance to adhesive and abrasive wear, albeit at the expense of increased brittleness and susceptibility to fracture. The use of vitamin E and other additives can potentially improve mechanical properties of PE. Despite this, metal-on-PE bearings still have the highest wear rates when compared to other combinations at 0.28mm/year of volumetric wear [20]. Using ceramic heads which have better scratch resistance than metal heads in these bearings have led to more favourable wear rates of less than 150 μm per year. This constitutes a 50% decrease in wear rates reported for traditional metal-on-PE bearings [91-93].
Hard-on-hard bearings currently include metal-on-metal and ceramic-on-ceramic. Metal on metal articulations are made of cobalt-chrome, providing high ultimate strength, superior biocompatibility, excellent corrosion resistance and reduced tendency to fretting. After an initial ‘wearing in’ phase, they have a self-polishing property and produce markedly reduced volumetric wear estimated at 2.5-5.0μm/year [91-93]. Furthermore, particles produced are of smaller size and number [94,95] which are thought to be less biologically active. However, increased levels of metal ions in blood, lymphatic tissues, urine and other tissues have been demonstrated with long term effects not yet elucidated [91]. Potential carcinogenic effects of metal ions are a concern [96] although no cases of neoplasia have been correlated directly with these articulations. There may be a potential for development of delayed-type metal hypersensitivities with MOM articulations [97]. Aseptic lymphocytic vasculitis associated lesions (ALVAL)[98] are characterized by the presence of extensive perivascular or diffuse infiltrates of both B and T lymphocytes [98-101]. large areas of necrosis may be caused by these reactions in spite of there being [102] minimal wear debris within these areas.
Ceramic-on-ceramic articulating surfaces combine properties of a high strength, scratch resistant material with very low coefficients of friction averaging 0.02, mimicking those of a normal joint. Their superior wettability and hydrophilic surfaces aid in lubrication when compared to an MOM articulation of the same diameter. This increases with head size, with the potential ‘holy grail’ of hydrodynamic lubrication in a hard-on-hard articulation. COC articulations reduce abrasive and adhesive wear as well as the number of wear particles produced that may take part in the loosening process or third-body wear [103]. Studies on cadaveric alumina heads showed hardly any change in surface roughness or roundness of the implant and scanning electron microscopy studies have shown hardly any deformation in surface compared to unused ceramic heads [103, 104]. Currently, COC articulations have the best wear profile, with annual wear rates of 0.5-2.5μm/year and volumetric wear averaging 0.004mm/year [105]. The particles produced are small and do not activate the osteolytic pathway in the same manner as PE does. The current reported failure rates of ceramic heads are less than 0.004% [22]. However, ceramics are expensive to produce, are unstable for certain geometric shapes and are brittle and if they do fail, they may fail catastrophically.
10.4. Prosthetic Head and Head-Neck Ratios
The prosthetic head plays an important part in generation of wear particles, a factor implicated in the loosening process. A smaller dimension head causes less volumetric wear due to having a smaller arc of motion than a larger head. The distance between two points that the head has to travel is also smaller for the same angle compared to a larger head (Fig. 4). A correspondingly larger head has to travel a greater distance for the same arc of motion and hence potentially generates more wear particles. However, linear wear in smaller heads is greater because the JRF is distributed over a smaller area. The head size is of particular relevance in hard-on-soft articulations such as metal-on-PE. Maximizing the femoral head-to-neck ratio using larger heads and smaller diameter necks improves head-neck ratios and excursion distances. In our experience, when considering a hard-on-soft articulation, a 28mm head probably represents a trade-off between volumetric and linear wear [106] and also produces a reasonable range of motion, although advances in PE hardness have allowed the use of 32mm heads without producing prohibitive amounts of wear debris. To avoid early impingement, it seems that the ratio between the femoral head and neck diameter should be over 2:1 [107].
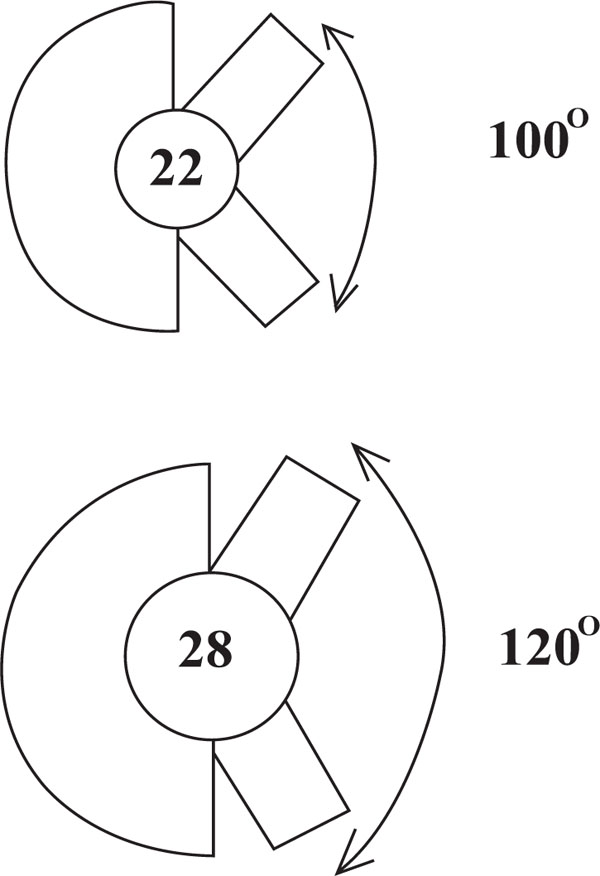 |
Fig. (4). Schematic diagram showing the effect of head size on primary arc range. |
With hard-on-hard articulations, the larger the head size the better due to the ever-increasing role of hydrodynamic lubrication and low volumetric wear. In our experience, this has a significant effect on stability not just due to improved head-neck ratios but also also due to the effect of suction on containment and an increased ‘jump gap’.
Constrained acetabular liners provide excellent stability but at the expense of a reduced primary arc range. In addition, large forces may be transmitted to the acetabular-bone interface, causing mechanical loosening. In our practice we occasionally use these sort of components for neck of femur fractures in the elderly, lower demand patients and patients with other conditions that may predispose to dislocation, for example neurological conditions.
10.5. Stem Shape
Cemented components are necessarily of lesser diameter than uncemented ones to allow room for the cement. In uncemented components, the stem diameters are greater in order to achieve maximum bone-implant contact, to encourage bony ingrowth. There are a variety of stem shape designs in use. Circular or elliptical sections have the least potential for bony attachment, except when a good initial fit is obtained [12]. Corners that cut into bone have been successful in reducing torsion when combined with certain surfaces. These achieve rotational stability as do the ones with longitudinal cutting flutes [108]. The larger uncemented stems rely on a wedge fit of the prosthesis into the proximal femur, including multiple contact points of the stem on cortical bone, the aim being to achieve an optimal fit both proximally and distally, to achieve axial and rotational stability [103]. In general, shorter stems are used for primary THRs whereas for revisions we may use longer ones. This is because of the large cavitary defects often present in the proximal femur in a revision setting, which require bypassing due to their poor capacity to take load. Biomechanical studies have shown that the stress pattern of tubular bone returns to normal at a distance of two bone diameters from the most distal defect [103]. Therefore, the length of the stem must bypass the most distal bone defect by at least 2-3 internal bone diameters to ensure stability. In addition, at least 4-5cm of intimate contact between the femoral isthmus and the implant is necessary [109] if distal fix is being relied upon for initial stability in a revision situation.
Dealing with cavitary defects in revision surgery is challenging and in our experience, impaction bone grafting using morselized allograft has proved to be a useful technique. Impaction bone grafting has the advantage of potentially reconstituting bone stock and allowing the surgeon to fashion the graft to the defect at the time of surgery [110] and can be used with the addition of cortical support in form of meshes, strut grafts and plates depending on the specific scenario presented [110].
Designs with a lateral flare attempt to maximize proximal fit and fill such that the length of the stem may be reduced [12]. In contrast to cemented stems that have smaller cross sectional areas, in uncemented stems the prosthesis is meant to fill the canal and hence must be of sizeable diameter and ideally achieve a greater than 90% fill [81]. Bending stiffness however, is proportional to the fourth power of the prosthetic diameter. Therefore, larger diameter stems may cause more stress shielding than smaller diameter ones [111, 112]. However, if a good proximal fit and fill is obtained and the stem is tightly wedged in the femur, high circumferential tensile stresses as well as up to 50% of the compressive stress component is produced [113], hence reducing bone loss. Using a material with a lower elastic modulus may permit the use of larger stems without the penalty of increased rigidity [114]. Stem stiffness is an important design variable that determines bone remodelling [115] and using a less stiff material has been shown to reduce stress shielding in the proximal part from 26-75% in the canine model [116].
10.6. Prosthetic Height and Offset (Abductor Tension)
Many of the prosthesis in current use have a modular head attached to the neck by a taper configuration. Cyclical shear stresses across the interface between the two parts intended to be statically fixed together may lead to fretting corrosion [117] which was a concern using stainless steel particularly in combination with ceramic heads. However, with the use of newer metal alloys, this problem is now less common.
One of the primary goals of THA is to position the primary arc range of the prosthetic hip in the centre of the functional range of motion required by the patient, in order to optimize the range of motion and reduce the chances of dislocation [19]. The position of the prosthesis in terms of neck length and lateral offset is critical and must provide adequate resting abductor tension for joint stability [75] and soft-tissue balancing is of increasing importance in THR [118]. Increasing the lateral offset increases the size of the abductor lever arm and abductor tension and reduces the JRF. However, increased torsional stresses on the stem and a potential for early loosening as well as trochanteric bursitis are potential problems with this. The vertical height or neck length also plays a part in appropriate abductor tensioning and also controls leg length.
In general, following THR, an average femoral offset of 45mm produces physiological loading [103]. However, in our practice we routinely use templating to determine the optimum height and offset for the individual patient. Lack of restoration of femoral offset leads to abductor weakness and limping [119]. A modular prosthetic system provides a simple way of adjusting neck lengths and offset in patients undergoing THA. A system with variable neck lengths or modular heads with variable internal recesses make simple adjustments to neck lengths and enable achievement of correct leg lengths. Some systems provide different stem offset sizes and one can also adjust these parameters to a certain extent by the depth of prosthetic insertion. We use the lateral decubitus position for our THRs and it has been postulated that leg length discrepancies are more likely to occur in this position. Therefore, in addition to preoperative templating, we use various techniques [120] recommended to prevent such discrepancies including assessing the patient’s feet in symmetrical knee flexion, measuring the height of the femoral cut from the top of the lesser trochanter and performing the osteotomy at the level determined by the preoperative template, assessing the relationship of the feet with the knees bent equally after trial reduction and the relationship of the greater trochanter with respect to the femoral head centre before and after femoral neck osteotomy.
10.7. Component Alignment
It is generally accepted that orientation of either component to an excessive degree may predispose to dislocation [121] irrespective of the surgical approach used to implant the prosthesis. Many prosthetic systems generally replicate the normal 10-15 degree femoral anteversion and studies have demonstrated a stem side anteversion angle of upto 15 degrees to be optimal [122, 123]. Optimum positioning of the acetabular cup is more controversial and does affect stability quite markedly. The surgical approach to the hip may affect the degree of anteversion used and surgeons may use a greater degree of anteversion using a posterior approach to prevent dislocation. For posteriorly implanted hips, 15-20 degrees of anteversion has been shown to result in excellent stability [124, 125]. The vertical orientation of the acetabular cup also affects stability and those inserted with a large coronal tilt angle are at increased risk of superior dislocation and generally, a 30-50 degree range for this angle is acceptable. In our practice we often use the transverse acetabular ligament (TAL) as a guide to acetabular orientation in our primary THAs as this is a fairly constant landmark in most native hips. The degree of acetabular coverage is also important for stability and long-term success. If less than 70% of the trial component is in contact with bone, we use a cortico-cancellous fragment cut from the femoral head and fixed into the cranial wall of the acetabulum with two screws to augment the superior wall. The use of posteriorly elevated acetabular liners may help prevent posterior dislocation in certain situations, albeit at the risk of posterior impingement. In some cases it may not be possible to control the anteversion of the stem that accurately, especially in cementless stems, and in this case the cup anteversion can be adjusted to provide a mean combined anteversion of 35 degrees with a safe zone of 25-50 degrees [126].
10.8. Modular Designs
The use of modular prostheses has partially overcome the highly unpredictable anatomy often encountered in revision situations. A modular prosthetic system provides a simple way of adjusting the vertical height and offset. Variable neck lengths or modular heads with variable internal recesses make simple adjustments to neck lengths. Some systems enable independent sizing of proximal and distal parts of the stem, and in some, the materials of the proximal and distal parts of the stem can be varied to reduce stress shielding. However, having sections of the stem with different elastic moduli may lead to stress risors in the stem, with a potential for failure.
Soft-tissue balancing is of increasing importance in THA and there have been sex differences demonstrated between women and men, with women having shorter femoral necks, thinner femoral shafts, lower cervico-diaphyseal angles, lower femoral offsets and greater femoral neck anteversion [5, 127-130]. Modular stems may have some theoretical advantages over monobloc stems in that they allow adjustment of the cervico-diaphyseal angle, lateral offset, neck anteversion, neck length and lower limb length independent of stem size and length [131]. In our practice, we use these components in complex situations with variation in different anatomical combinations, for example in high grade DDH (thin shaft, long neck, high offset), large shaft/short neck/high offset, and in situations where there are other mismatches between stem size and neck length or offset and where there is an excessive amount of anteversion. These components have however been associated with a failure rate of about 0.027% [131].
10.9. Custom Designs
Custom designs are mainly used in revision situations because of the highly distorted and variable anatomy of the femoral canal and acetabulum due to to large cavitary defects in these situations. Three-dimensional geometry of the femoral canal and acetabulum is usually determined either by serial radiographs [132] or CT reconstruction [133, 134], or direct shape determination at surgery [135, 136] and these implants have shown some success in terms of stability and bone preservation in revision situations [137]. In situations where uncemented components are to be used and the anatomy is atypical, customized components designed using computer aided design and computer aided manufacture (CAD-CAM) to maximize the ‘fit and fill’ in the proximal femur at the time of implantation can be used, thereby providing immediate stability to the implant [138, 139]. The strain patterns in the proximal femur have been found to be closer to normal using CAD-CAM designed prosthesis compared with other bone mass-sparing prostheses [138] and have also demonstrated clinical success in situations of atypical anatomy [140].
10.10. The Future of Total Hip Arthroplasty
THRs are being performed in an increasingly younger and more active patient age group compared to earlier years. The main cause of failure in these patients remains loosening due to osteolysis and the focus in future is going to be on extending the durability and survivorship of these components in a younger patient age group.
There may be future interest and developments in the pharmacological inhibition of the osteolytic response as well as the developement of novel materials or surfaces that may enhance bony in growth onto implants.
There is currently on going controversy regarding the use of minimally invasive surgery in THR, with it’s proponents saying it causes less tissue trauma and blood loss and less instability and faster recovery. However, others have reported a greater degree of tissue damage, femoral fracture and that nerve damage and component malpositioning is greater, in addition to having a steep learning curve with no significant differences in outcomes three months after the operation compared to conventional THA [141-143]. This controversy should be resolved in the next few years as more data becomes available on outcomes as well as experience with the techniques increase.
Optimum positioning of the femoral and acetabular components have led to various navigation systems being developed whose role in day to day THR is yet to be determined [144]. At present, navigation techniques are not recommended as a routine procedure [105]. However, these systems have been shown to be useful in some studies, particularly with positioning of the acetabular component [145] and the use of navigation may become more widespread in conjunction with minimally invasive techniques for THA.







