All published articles of this journal are available on ScienceDirect.
Complications after Spinal Surgery in Patients with Parkinson’s Disease
Abstract
Background:
Several studies have shown that spinal surgeries in patients who suffer from Parkinson’s Disease have a high rate of complications. These patients often need revision surgery.
Objective:
This is a retrospective study involving 21 patients with Parkinson’s Disease. This study aimed to examine the complications after spinal surgery.
Methods:
We studied 21 patients with Parkinson’s Disease retrospectively, who had undergone a previous operation by the same surgeon between 2004 to 2019. There were 11 females and 10 males. The mean age was 71.9 years (range, 52 to 85). However, the initial diagnosis and types of surgery were different. The mean time of follow-up for each patient was 3.6 years (ranging from 2 to 8 years).
Results:
Most of the patients had a post-operative complication within a period of three years. Τhe most common complication was kyphotic deformity and camptocormia. Twelve patients (57.1%) underwent revision surgery, and three patients (14.2%) denied treatment. In four patients (19.04%), kyphotic deformity or stooped posture remained. Only one patient (4.7%) presented with no complication in a follow-up of 8 years.
Conclusion:
Patients with Parkinson’s disease have a high rate of complications after spinal surgery and often need revision surgery. For this literature review, the overall number of patients was 502, and the mean revision rate was 43.6%. The surgeon must inform patients of possible complications, and a thorough post-operative observation must be implemented.
1. INTRODUCTION
Parkinson’s Disease (PD) is a progressive disorder that affects dopaminergic cells of the substantia nigra. Parkinson’s disease is the second most common neurodegenerative disease after Alzheimer’s disease. Parkinson’s disease is an age-related disorder; its prevalence is estimated at 1% of the population up to 60 years old and has a wide spectrum of symptoms, such as bradykinesia, tremor, and rigidity, flexion of the trunk, hip, and knees. This disorder leads to abnormal loads on the spine [1, 2].
It is very common for these patients to develop spinal malalignment in sagittal and coronal planes, which affects their quality of life [3]. Several spinal deformities are associated with PD, such as camptocormia, bent spine syndrome, anterocollis (dropped head syndrome), and Pisa syndrome (lateral flexion and axial rotation of the trunk) [4]. Camptocormia is the excessive flexion of the thoracolumbar spine due to muscular weakness, and the bending angle must be greater than 45 degrees. This condition is usual among PD patients [5]. In addition, patients with PD are fragile, having a high rate of falls and osteoporosis [6-8].
Spinal surgery improves deformity of the spine in these patients [9]. Moreover, a lot of studies have shown that surgical treatment of various diseases of the spine in PD patients is associated with a large percentage (from 20-80%) of postoperative complications that make revision surgery necessary [9-11]. Surgical correction should be considered for patients with a good prognosis [12, 13]. The purpose of the present case series is to assess the number and type of complications.
2. PATIENTS AND METHODS
Twenty-one PD patients who underwent spinal surgery between 2004 to 2019 were retrospectively studied. Comorbidities from patients' charts, imaging studies, and surgery reports were recorded. All patients underwent whole-body radiographs. Supplementary images of the entire spine to assess spinal stiffness were taken in lateral right-bending and left-bending positions.
In all patients, transcranial motor-evoked potentials, somatosensory evoked potentials, and free-running electromyography of the lower extremities and evoked electromyography with pedicle screw stimulation were used. Implant position, sagittal balance, fusion and progressive deformity, and/or implant failure or revision surgery were recorded. Comorbidities other than PD were also recorded. Hypertension was present in 3 patients, hypothyroidism in 3, and one patient presented with chronic atrial fibrillation and hypertension. All patients had a written informed consent in order to be included in this study. The study was approved by the Scientific Committee of the institutions involved.
3. RESULTS
The mean age of the patients was 71.9 years old (52 to 85). The mean time of follow-up was 3.6 years (ranging 2 to 8 years). The underlying pathology that led initially to surgical treatment was different among the patients. Spinal segments involved the lumbar spine in 11 patients, the thoracolumbar spine in 9, and the cervical spine in one patient.
There were 10 males and 11 females. Four patients had kyphotic deformity after vertebral fracture, three had lumbar degenerative scoliosis, ten had lumbar stenosis, two had a vertebral fracture, one was treated for degenerative disc disease, and one patient had cervical spine stenosis. Patients were treated either with spinal fusion, laminectomy, or vertebrectomy for spinal decompression or kyphoplasty with or without spinal fusion (Table 1).
In our study, 20 patients out of 21 had a worsening of symptoms within three years post-operatively. One of the patients initially treated with fusion from L2-S1 had a complication six months post-operatively. He had proximal junctional kyphosis but good hardware alignment. He refused further surgical treatment, and he presented with a flat back three years post-operatively. Another patient sustained a compression fracture above the level of fusion one year post-operatively. Only one patient who was initially treated for lumbar stenosis had no complication 8 years after surgical treatment. Four patients underwent an extended spondylodesis from the upper thoracic spine down to the sacrum and iliac bones of the pelvis. These patients at the long-term follow-up presented clinically with insignificant loss of correction, and thoracic kyphosis and lumbar lordosis were preserved close to normal. Post-operatively, all patients were subjected to intensive physical therapy in order to delay the onset of postural disorders. The mean time of each patient follow-up was 3.6 years (ranging 2 to 8 years).
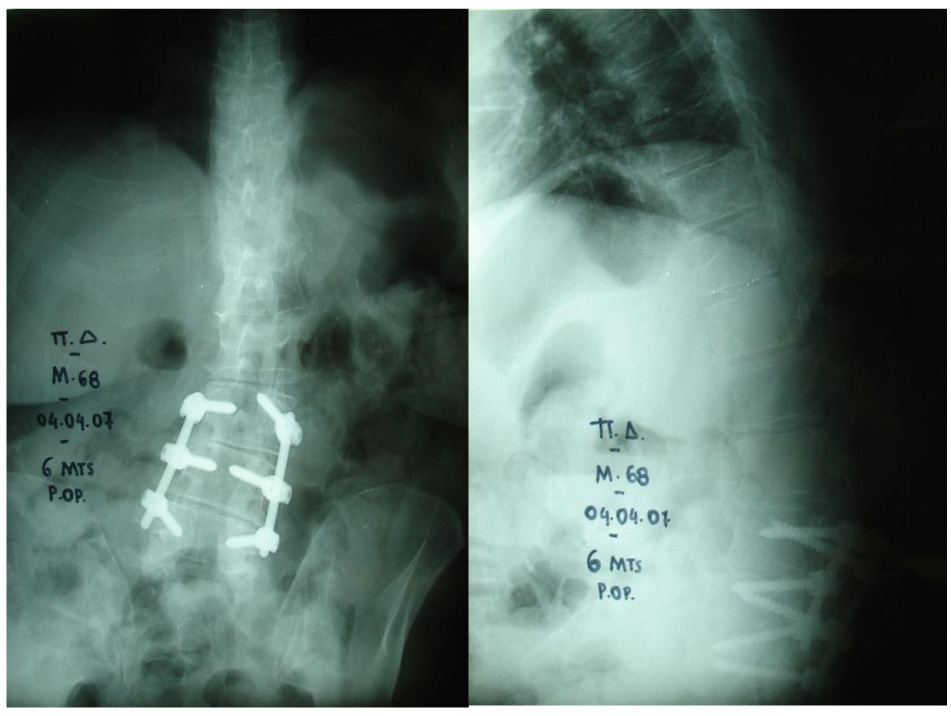
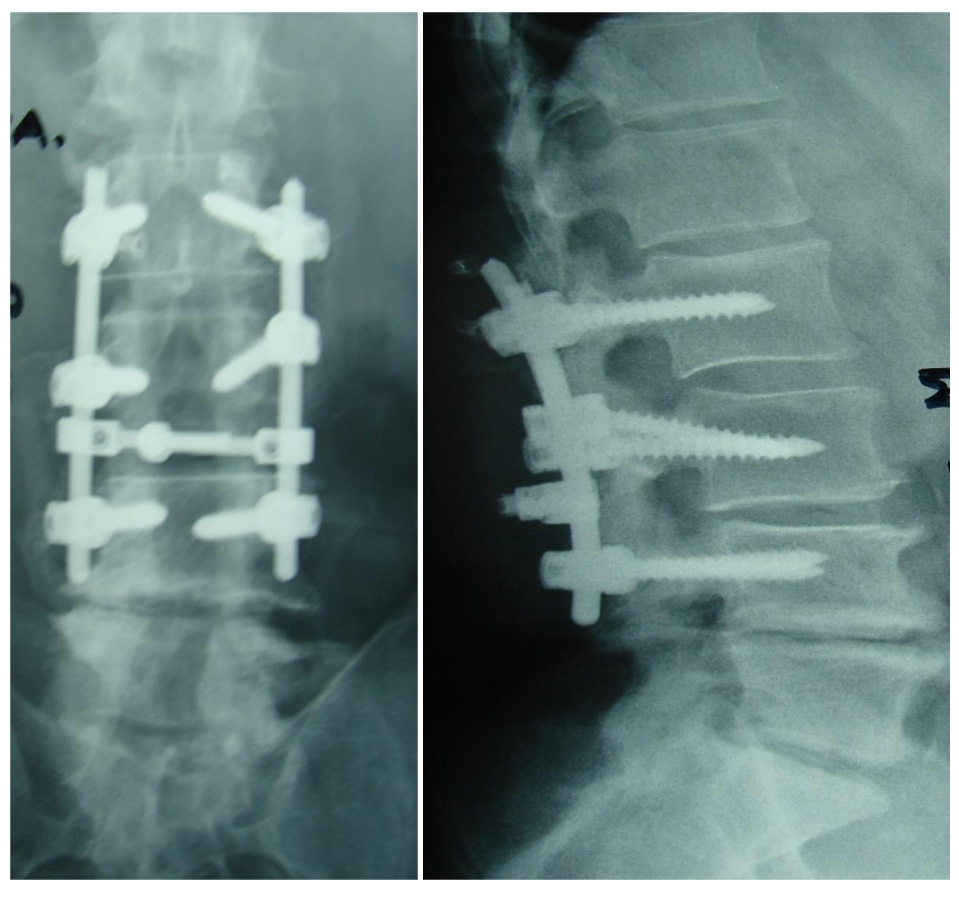
No neurological complication during surgery or the immediate post-operative period of the first three weeks was observed. Twenty out of 21 patients were presented with a complication at a mean of 1.6 years (range 2 months to 3 years) after surgery. Τhe most common complications were kyphotic deformity and camptocormia (7 patients/33.3%) (Fig. 1). Twelve patients (57.1%) underwent revision surgery, and three patients (14.2%) denied treatment. Kyphotic deformity or stooped posture remained in four patients (19.04%). Only one patient (4.7%) presented with no complication in a follow-up of 8 years (Fig. 2). One patient did not appear for follow-up. Table 1 shows the patients’ data. The reported rate of complications in published studies is shown in Table 2. In this literature review, the overall number of patients was 502, and the mean revision rate was 43.6%.
| Patient | Age | Gender | Diagnoses at First Presentation | Index Surgery | Time of Complication Development | Type of Complication Development | Time to Revision Surgery | Revision Surgery | Remarks | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | M | VCF T5, T9, L5 | Kyphoplasty at all three levels | 16 months | Marked kyphosis T7-T8 | 18 months | Fusion T2-T7 | - | |
| 2 | 74 | M | Lumbar scoliosis | Levels of fusion T12-S1 | 14 months | ASD, Proximal junctional Kyphosis | - | - | Declined further surgery, two years post-operation died due to heart problems | |
| 3 | 52 | F | Kyphosis, VCF T12 | Kyphoplasty at T12 vertebra | 2 months | VCF L1 | 3 months | Fusion T9-L4 | Pull out L2-L3 | |
| 4 | 68 | F | Kyphosis, VCF T12 | Fusion T9-L5 | 2 years | Camptocormia | - | - | Worsening over time | |
| 5 | 65 | F | Kyphosis, VCF L1 | Fusion T7-L5 | 18 months | Camptocormia | - | - | Worsening over time | |
| 6 | 72 | M | Lumbar stenosis | Laminectomy L3-L5, fusion L2-L5 | 18 months | Pull-out L2, ASD L1-L2 | 20 months | Fusion T12- L5 | Stooped posture remains | |
| 7 | 70 | F | Lumbar stenosis | Fusion T12- L5 | 12 months | VCF T6,T12 | 12 months | Kyphoplasty | VCF T7, T9 2 years post op | |
| 8 | 77 | M | Lumbar stenosis and scoliosis | Laminectomy L3-L5, fusion L3-L5, interspinous implant L2-L3 | 24 months | - | - | - | Flexion contracture hips and knees | |
| 9 | 68 | F | Disc herniation L3-L4, L4-L5 | Laminectomy L4- L5,interspinous implant L3-L4 | 24 months | ASD L2-L3 | 24 months | Laminectomy L2- L3, fusion L2-L5, interspinous implant L1-L2 | Stooped posture 3 years post-op | |
| 10 | 79 | F | Kyphosis, VCF L1 | Spinal Fusion T9- L3, Transpedicular vertebrectomy L1-Harms cage | 27 months | Pull-out L2, L3 screws | - | - | Fused in a stooped posture | |
| 11 | 67 | M | Lumbar stenosis | Fusion L2-L5, Laminectomy L3 | 6 months | Destabilization above spondylodesis L3-L4-L5 | - | The patient declined further treatment | - | |
| 12 | 79 | F | VCF | Fusion T1-L5 | 2 years | Proximal junctional kyphosis | - | Refused further treatment | - | |
| 13 | 85 | M | Lumbar stenosis | Laminectomy at the L2-L3,L3-L4,L4-L5, unilateral facetectomy of L3-L4 level | 2 years | New stenosis, facet cysts | 2 years | Spondylodesis T5-S1 | - | |
| 14 | 77 | F | Lumbar stenosis | Fusion L2-S1 | 4 months | Lumbar instability | - | - | Lost to follow-up | |
| 15 | 68 | M | Lumbar stenosis | Interspinous spacer of L4-L5 levels | 3 years | New stenosis | 3 years | Fusion L1-L5 | A year after fusion, new stenosis, and RE-revision L1-L5 | |
| 16 | 76 | M | Lumbar stenosis | Fusion T10-S1 | 10 months | Compression fracture above spondylodesis | 1 year | Fusion T9-S1 and discectomy | - | |
| 17 | 65 | F | Lumbar stenosis, scoliosis | Discectomy at L4-L5 level and foraminoties at L3-L4/L4-L5 | 3 years | Post-laminectomy lumbar instability | 3 years | Fusion L2-L5 | 4 years later, the spondylodesis extended to the sacrum | |
| 18 | 67 | F | Scoliosis, | Fusion T1-L4 | - | - | 1 year | The fusion extended to the sacrum due to instability L4-L5 | 3 years post-op: camptocormia, no hardware failure | |
| 19 | 73 | F | Lumbar stenosis, scoliosis | Laminectomy L3-L4-L5 | - | Scoliosis | 3 years | Fusion L2-L5, implants | 5 years after surgery: camptocormia, scoliosis | |
| 20 | 66 | F | Lumbar stenosis | Fusion L3-L5 | - | - | - | - | Six years post-op: no complication | |
| 21 | 71 | M | Cervical stenosis | Anterior fusion | 1 year | - | 1 year | Posterior fusion | Enhance stability | |
| Author | Patients | Revision Rate | Remarks Due To |
|---|---|---|---|
| Bouyer et al.[18] | 48 | 42% | Hardware complications |
| Schroeder et al.[21] | 96 | 20.8% | Early complications related to infection |
| Babat et al.[10] | 14 | 85.7% | Technical complications |
| Koller et al.[15] | 23 | 33.3% | High rate of infection |
| Sarkiss et al.[16] | 95 | 45% | N/A |
| Scenema et al.[35] | 18 | 33% | Follow-up 2 years only |
| Bourghli et al.[24] | 12 | 50% | Long spinal fusion T2-sacrum |
| Moon et al.[25] | 20 | N/A | Compared to no PD patients |
| Wadia et al.[13] | 2 | 50% | Two cases of camptocormia |
| Kaspar et al.[23] | 24 | 21% | Mean nineteen months follow-up |
| Sheu et al.[39] | 66 | 29% | Analysis of risk factors |
| Westerman et al.[37] | 36 | 27.8% | Case control study |
| Liu et al.[36] | 25 | 80% | Segmental instability |
| Yamato et al.[38] | 22 | 36% | Within 3 years |
| Sapkas et al.[34] | 21 | 57.1% | Revision within 3 years |
| Total | 502 | 43.6% |
4. DISCUSSION
Patients suffering from PD and who are submitted to spinal surgery have a high rate of complications. The most common complication reported is instability at the level above the spondylodesis due to adjacent spinal segment degeneration, screw pull out, flat back, and camptocormia [12-16]. Revision spinal surgeries are also a very common phenomenon; therefore, close follow-up of these patients is crucial [17]. According to Oh et al., patients with PD have a high prevalence (42%) of sagittal spinopelvic malalignment [14].
Surgical complications can be divided into early and late ones. Early complications related to Parkinson’s systemic impairment are seen in the immediate post-operative period. In a recently published multicentric study, 42% of 48 patients who underwent a long fusion from the upper thoracic spine to the sacrum or pelvis required revision surgery. The authors pointed out that the main complications were pseudarthrosis and junctional kyphosis [18].
In a study by Koller et al., 23 PD patients suffering from various spinal disorders were surgically treated. Fifty-two percent of the patients presented with a complication, and 33% of them had revision surgery. However, a high rate of satisfaction among patients of 74% was reached with the clinical results. The authors stated that restoration of the sagittal balance is crucial in order to achieve successful results. This observation can be attributed to the fact that PD patients do not require the same degree of restoration of the sagittal alignment in order to enable a line of sight safe enough to walk, and also they have reduced mobility and lower daily functional activities than the general population [15].
In a study by Torsney et al., the authors found that osteoporosis was a risk factor in a ratio of 2.61 in PD patients compared to healthy controls [19]. Furthermore, a lower Bone Mineral Density (BMD) and an increased fracture risk are also reported. Vitamin D deficiency and anti-Parkinsonian drugs can be involved in the reduced BMD [20].
Schroeder et al., in light of their findings, recommend that when treating a patient with PD, the most critical point of discrimination is the severity of the disease [21]. The modified Hoehn and Yahr staging scale is used for PD. This scale includes stage 1, unilateral involvement only; stage 2, bilateral involvement without impairment of balance; stage 3, mild to moderate bilateral disease, some postural instability, and physical independence; stage 4, severe disability but still able to walk or stand unassisted; and stage 5, wheelchair-bound or bedridden unless aided [21]. For patients with a modified Hoehn and Yahr stage of >3, surgery should be performed only in cases with myelopathy due to high complications risk. However, in stage <3, other comorbidities of the patients should be evaluated. If no major risk factors are present, then the patient’s spine pathological condition should be evaluated. Overall, the surgical risk for the patient is higher than that for the general population [22].
Kaspar et al. assessed the post-operative complications of all types of spinal surgeries in PD patients and found a revision rate of 4/24. They concluded that the complication rate in PD patients was comparable to that of the normal population. Furthermore, the functional damage and symptoms directly related to the spinal disease had been masked by PD, causing diagnostic difficulties, especially for cervical arthritic myelopathy [23].
Babat et al. retrospectively studied 14 patients with PD who had spinal surgery. They noted a high rate of surgical revision (86%). They suggested the segmental instability at the level of surgery and kyphosis at the junctional levels as primary causes of this high revision rate [10]. This is in accordance with the findings of our study that the main causes of complication seen were kyphotic deformity and camptocormia (7 patients/33.3%). Also, the revision rate was also high (12 patients/57.1%).
Adjacent segment degeneration with Proximal Junctional Kyphosis (PJK) has been widely described after posterior procedures. The etiology of PJK is probably due to various factors among these patients, including the iatrogenic effect of the fusion, age-related osteoporosis, disc degeneration, and neuromuscular disease [24].
Bourgli et al. and Koller et al. insisted that if spinal surgery is indicated in patients with PD, the restoration of spinopelvic balance with a focus on lumbar lordosis and global sagittal alignment is required. Statistical analysis revealed that patients with notable postoperative or follow-up sagittal imbalance (sagittal vertical axis (SVA)>10cm) had a significantly increased rate of revision surgery performed or scheduled. Patients who underwent surgery were more likely to have post-operative or final sagittal imbalance [15, 24].
Poor clinical outcome is related to the natural progression of the pathology [18, 25]. However, risk factors should be considered in selected patients who might benefit from the surgical intervention. Sarkiss et al. showed that poor outcome was associated with older age, thoracolumbar kyphosis, osteoarthritis of the hip, and increasing level of camptocormia. Risk factors related to the surgery were postoperative SVA greater than 5 cm, inadequate sacropelvic fixation, and poor fusion level selection [26]. Another review by Galbusera et al. concluded that poor outcomes related to a high rate of complication and revisions are usual, but the majority of patients are satisfied with their new quality of life [27]. In addition to low bone quality, postural instability, motor disorders, and autonomous nervous system dysfunction play an important role in fracture risk after a fall [27]. On the other hand, it is worth noting that all of the patients are of progressive age, and they are often presented with comorbidities [28, 29] and impairment of visual-spatial abilities of parietal cortices bilaterally in Pisa syndrome [30]. This fact is highlighted in a study by Baker et al., who reported an increased risk of cardiac, pulmonary, and haemorrhagic complications in PD patients compared to non-PD patients who underwent spinal surgery [31]. According to Vaserman et al., patients with PD have a high osteoporosis rate [6]. In combination with muscular dysfunction, osteoporosis contributes to fusion failure [31-33]. In a study by Sapkas et al., it was pointed out that close follow-up of PD patients with a complication is crucial [34]. Scenama et al. noticed no association between C7 plumbline and last follow-up in the Oswestry Disability Index (ODI) [35]. In such cases with osteoporotic bones and loss of function of the spinal extensor muscles, directly related to the disease and the age-associated fatty degeneration (steatosis), long arthrodesis by a posterior approach extending from T2 to the sacrum is indicated.
Our personal opinion is that careful pre-operative planning, especially to evaluate the sagittal balance of the spine, is very important. Whenever this factor is not taken into account, the problem of spinal destabilization has quickly appeared. The sagittal balance is found to be a more significant factor in comparison to coronal balance. However, even when the sagittal balance is evaluated pre-operatively, this does not exclude the destabilization of the spine above the last level of spondylodesis. This is in accordance with similar observations referred above. However, better results were obtained when the spondylodesis was extended from the upper levels of the thoracic spine down to the sacrum and iliac bones of the pelvis. In cases where this type of operation was performed initially, the loss of correction at the long-term follow-up was not significant. On the contrary, when short spondylodesis was performed at the lumbar spine, following laminectomies, limited laminectomies, and /οr discectomies, the results were unsatisfactory. Immediately post-operatively, the balance deteriorated, especially over the fusion levels. It is difficult to provide information regarding the degree of osteoporosis-osteopenia of the patients, as they were not subjected to detailed pre-operative DEXA scanning of the spine and hips. However, in cases when plain x-rays, MRI, and CT scans showed osteopenia-osteoporosis or collapse of the vertebra due to osteopenia and/or osteoporosis, the surgery was modified in order to increase the stability of the vertebrae and the hardware of the spondylodesis. Collapsed vertebrae were re-enforced with methyl-methacrylate (kyphoplasty) and stabilized with cannulated screws for osteoporosis.
It is significant to note that all the operated patients returned to normal daily activities customized for their age and general health status. All of them were under neurological supervision with the appropriate medical therapy for PD. One of the patients who underwent long stabilization of the deformed spine had substantial improvement in both standing and walking ability following brain surgery. It is well understood that the strength of the musculoskeletal system is of the utmost importance for the stability of the body. In practice, it is pretty difficult for these patients to be involved in gymnastic activities to obtain, at least, the ability to be able to stand in an upright position with an acceptable level of pain.
Many of our patients underwent various types of operations at the lumbar spine for discectomy or laminectomy for stenosis, with or without short fusions. The number of patients included in this study is small, and there are many sub-categories. Therefore, definite opinions are hard to extract. None of the patients resembled the other, and factors such as variations in the initial cause of surgery, differences of body stature, and extent of PD involvement differentiate the type of operative treatment.
According to our observations, before opting for operative treatment for a patient with PD, we suggest that surgeons should consider: a) pre-operatively: i) total body x-rays to evaluate the sagittal and coronal balance of the spine; ii) the presence of osteoporosis/osteopenia and the appropriate treatment; iii) the neurosurgical status of the PD and the possibility of relevant intervention; iv) the medication for concomitant co-morbidities, and v) previous surgeries for spinal diseases. b) intraoperatively: i) avoid destabilization of the operated lumbar levels by not excising the facets; ii) spondylodesis using cannulated screws for osteoporosis and injecting methyl-methacrylate into the vertebral bodies, as it is significant to reinforce the end vertebrae; iii) in order to restore the sagittal balance, long spondylodesis should be extended from the upper thoracic vertebrae to the sacrum-pelvis/iliac bones; iv) copious amount of auto or allographs are necessary to enhance fusion, and v) to increase the stability of the spondylodesis, screws should be inserted in every spinal level, and double rods at the lumbar spine should be placed, in order to avoid implants failure. c) post-operatively: i) an intense rehabilitation program should be followed in order to increase muscle strength and the ability to stand and walk; ii) compliance of their recommended medical treatment for PD, and iii) neurosurgical treatment should be provided in selected cases. In our opinion, all the aforementioned steps in PD patients should be followed in order to offer the best possible solution and eliminate complications.
CONCLUSION
Patients with PD often undergo spinal surgery mainly due to kyphotic or other deformities. These surgeries have a high rate of late complications. Although poor clinical outcomes related to a high rate of complications and revisions are frequently reported, most of the patients are satisfied by surgery and report better quality of life compared to the pre-operative period. Therefore, careful pre-operative planning needs to be implemented for the correct selection of patients and the fusion level. Furthermore, it is necessary to maintain a close post-operative follow-up even though the results are disappointing, and revision surgery is often needed. As the evidence amasses, it is becoming clear that PD patients are a high-risk subgroup. Early preventive physical therapy may be able to delay the onset of postural disorders but will not prevent their progression.
LIST OF ABBREVIATIONS
| PD | = Parkinson’s Disease |
| BMD | = Bone Mineral Density |
| PJK | = Proximal Junctional Kyphosis |
| ODI | = Oswestry Disability Index |
| SVA | = Sagittal Vertical Axis |
| VCF | = Vertebral Compression Fracture |
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the scientific committee of the institution KAT General Hospital of Attica, Greece (Ethical code# 16189).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients provided written informed consent.
AVAILABILITY OF DATA AND MATERIALS
The data supporting the findings of the study is present within the manuscript.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


