All published articles of this journal are available on ScienceDirect.
Intervention versus Observation in Mild Idiopathic Scoliosis in Skeletally Immature Patients
Abstract
Introduction:
Observation is the treatment of choice for idiopathic scoliosis with Cobb angles between 15 degrees - 20 degrees in growing children. This passive approach does not address the anxiety of the patient and the stress of the parents. In this paper, we attempt to identify skeletally immature patients with mild scoliosis curvatures that are more at risk of progression and propose possible intervention for this group of subjects.
Methods:
The literature was searched in Pubmed, and additional references were searched manually in the literature.
Results:
Many studies have shown that low serum 25[OH]D level, bone mineral density (BMD), and body mass index (BMI) are related to the curve severity or progression of the curve.
We suggest that skeletally immature patients (< Risser 2) with mild curves be divided into two groups, viz. Group O (observation) with a lower risk of progression, and Group I (intervention) with a higher risk of curvature progression. We propose early intervention for the latter group.
It is suggested that pre-menarcheal, skeletally immature patients with mild idiopathic scoliosis, and low vitamin D, BMD, and BMI should be treated. Also, asymmetric foot biomechanics should be addressed, although nutrition and foot orthoses are regarded to have no role in the management of idiopathic scoliosis. The outcome of early intervention may be utterly different from late treatment when the curvature becomes more structural, and the patient more skeletally mature.
Conclusion:
Research is required to prove if the intervention is clinically indicated.
1. INTRODUCTION
Idiopathic scoliosis is defined as a 3D spinal deformity, with lateral curvature of the spine ≥ 10 degrees with no identifiable causes. Treatment of the condition varies with the magnitude of curvatures and the remaining growth potential and includes observation, Physiotherapeutic Scoliosis Specific Exercises (PSSE), bracing, and surgery [1]. Of these, PSSE and bracing have the first level of evidence. Observation and surgery, although advocated as treatment, do not have a high level of evidence [1].
Observation has been stipulated as the treatment of choice for Juvenile Idiopathic Scoliosis (JIS) and Adolescent Idiopathic Scoliosis (AIS) with angles between 15 degrees - 20 degrees in growing children [2], as the trajectory of mild curvature is chaotic. Some curvatures progress, some remain stable, and some resolve [3, 4]. Patients are thus followed up regularly until the trajectory of curvatures development becomes clear [3]. Early intervention for mild scoliosis is generally regarded as over-treatment. When the Risser is 0 and 1, patients are usually followed up every three months [2, 5]. When the curves progress >5 degrees between visits or become > 20 degrees - 25degrees, PSSE and bracing are indicated [2], [5].
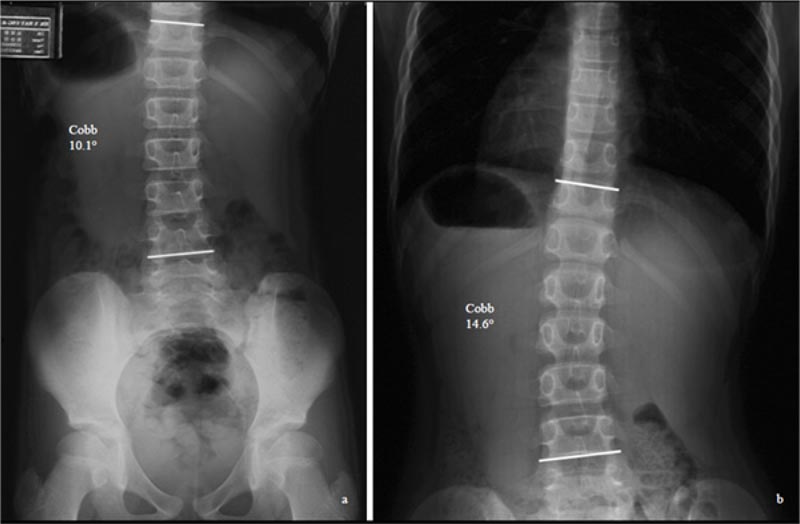
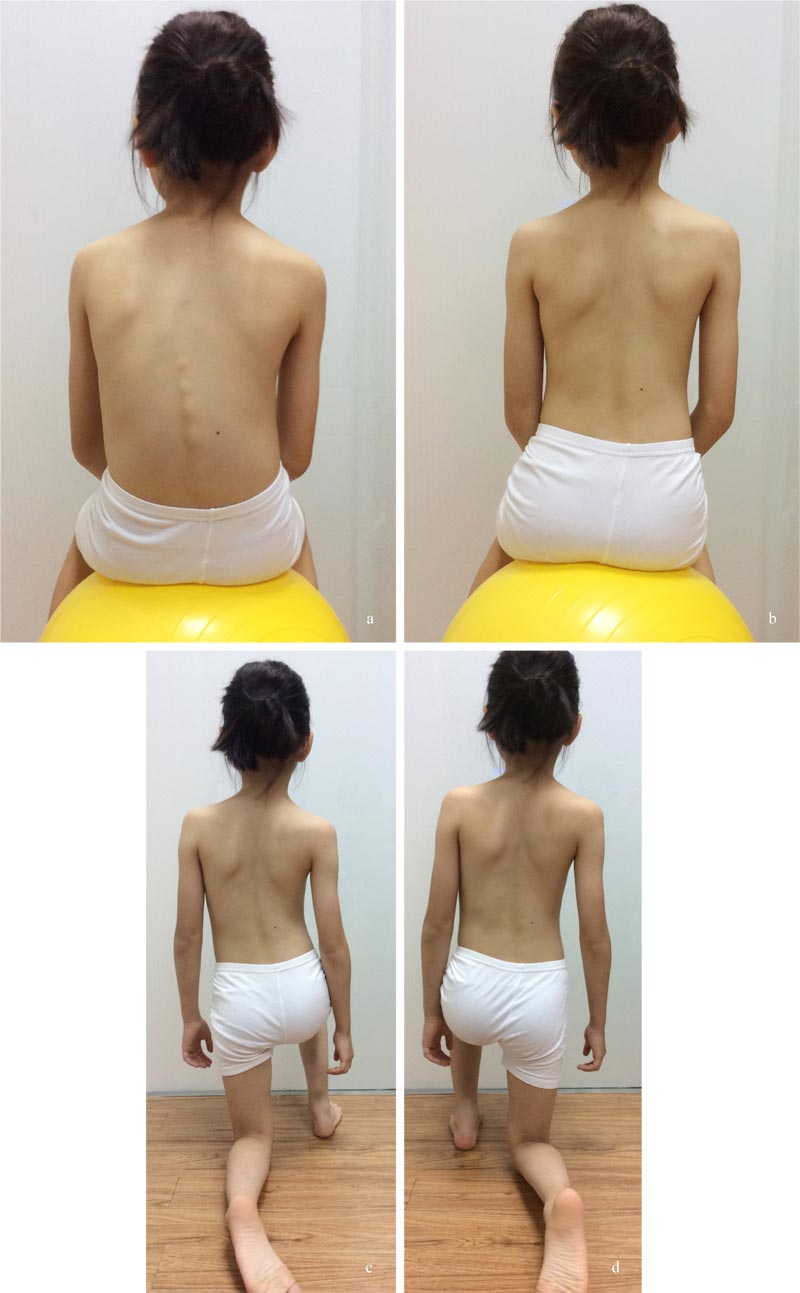
This passive approach does not address the patient's anxiety [6] and the stress of the parents, who worry about the progression of curvatures (Fig. 1). Once the curves progress, the non-surgical treatment prescribed will be much
More time-consuming and expensive. The high (40%) drop-out rate in the observation group in observation versus bracing studies [7] suggests that the parents may opt for active intervention instead of being observed for their children [8]. The clinicians may be seeing the condition from a different perspective from the parents.
The current study reviews the literature to see if it is possible to identify skeletally immature patients with mild scoliosis, who are more at risk of progression, and to propose intervention for this group of subjects.
2. MATERIALS AND METHODS
Papers were searched in PUBMED, using the Boolean search operators: (“Prognostic factors” OR “Risk of Progression” OR “Natural History”) AND “adolescent idiopathic scoliosis” NOT “surgery.” All the abstracts of the papers retrieved were reviewed. Non-English articles and those related to genetics, screening, cardio-pulmonary impact, long-term effects, and pathogenesis were excluded. The articles referenced in the reviewed literature during the search were also examined to see if they are appropriate for inclusion in the discussion.
3. RESULTS
The search produced 32 articles. After the exclusion, only 18 papers were left. Additional related articles were manually searched from the reviewed articles and included when relevant.
Results revealed that some risk factors are associated with the progression of mild curvatures in skeletally immature patients. These include an initial Cobb angle severity >25degrees, osteopenia, <13 years of age at diagnosis, pre-menarcheal, skeletal immaturity [9], low serum 25[OH]D level [10, 11], and low body mass index (BMI) [12, 13]. It is of interest to note that the serum level of 25[OH]D and BMD are inversely correlated to the Cobb angle [10]. Osteopenia [14] and body weight [15] have also been shown to be an independent and prognostic factor for the progression of curvatures in AIS patients.
For intervention, we could not find any studies that have investigated the effects of improving BMI, BMD, and vitamin D3 supplementation on scoliosis curvatures. Yet, custom foot orthoses were reported to improve mild curvatures in juvenile patients with curves below 25 degrees [16].
4. DISCUSSION
4.1. Risk Factors of Curves Progression
There are, at present, no objective ways to know if a particular curvature would progress, despite that some risk factors for curvature progression have been reported (Table 1). Of the ten predictors of curvatures progression reported by Noshchenko et al. (2015) [9], the only relevant clinical predictors of progression for the present discussion would be residual growth potential, age <13 years, and osteopenia.
Many studies have shown that AIS patients have significantly lower 25[OH]D level than control subjects [10] [17]. Also, BMI is significantly lower in AIS patients than healthy controls [12, 13, 15]. Interestingly, serum 25[OH]D level [10], BMD [18], and BMI [15]were reported to be inversely related to the Cobb angle. Bodyweight was identified as an independent predictor of curve magnitude in male AIS patients [15]. Recently, Kim et al. (2020) reported that underweight is associated with scoliosis in Korean female adolescents [19].
Female gender is a risk factor for progression. The reported female to male ratio ranges from 1.5:1 to 3:1, and increases substantially with increasing age. The prevalence of curves with higher Cobb angles is significantly higher in girls than in boys, rising from 1.41:1 in curves from 10 degrees to 20 degrees to 7.2:1 in curves >40 degrees [20].
Different curve types progress differently. Of the different curve types, Lenke 1 curve (thoracic curve) is the most progressive. Zapata et al. (2019) followed the progression of mild curvatures (15 degrees -24 degrees) in patients, with Risser 0 and 1. Of the cohort of 302 consecutive patients, 24 (7.9%) cases progressed to >45 degrees. The Lenke 1 curve was the most common surgically treated type (58%), suggesting that of all the curve patterns, the main thoracic curve pattern is the most likely to progress [4].
4.1.1. Serum Vitamin D Level
Many studies have compared the serum 25[OD]D levels in AIS patients with age-matched healthy controls. They have reported that the serum 25[OD]D level of AIS patients is significantly lower than that of the controls (Table 2) [10, 11, 21]. Balioglu et al. (2017), in a retrospective study, evaluated the serum 25[OD]D level of AIS patients and age-matched controls. Results showed that the serum vitamin D level of AIS patients is significantly lower than that of controls [10]. Similar findings were reported by Gozdzialska et al. (2016) [11]. Balioglu et al. (2017) reported that the serum 25[OH]D level is negatively correlated with the Cobb angle (p<0.026), yet Silva et al. (2017) found no relationship between the serum 25[OH]D level and the Cobb angle [10, 21].
4.1.2. Bone Mineral Density (BMD)
Adolescent idiopathic scoliosis patients have been found to have lower bone mineral density than healthy controls [22-26]. Osteopenia is a generalized phenomenon [23, 25, 26], involving not only the lumbar spine but also the radius and tibia. Lee et al. (2005) studied the BMD and curve magnitude in 919 AIS patients during the peri-pubertal growth period and reported that the BMD was inversely correlated to the curve magnitude [27]. Patients with lower bone mineral density generally had larger Cobb angle, whereas patients with normal BMD had smaller Cobb angles [27]. Subsequent studies by the same group supported the findings [18, 25, 10]. Osteopenia is a significant prognostic factor for curve progression [18].
| Gender - female |
| Curve type – thoracic major (Lenke 1 curve) |
| Risser -0 – 1 |
| 25[OH]D level – insufficiency or deficiency |
| Bone mineral density – osteopenic |
| Body mass index - underweight |
| Asymmetric foot biomechanics |
| Study | Samples (n, age) | Ethnicity | Findings | Recommendation |
|---|---|---|---|---|
| Bagliou et al. 2016 | 229 AIS (14.7) vs 389 age-matched controls (13.9) | Turkish | Vit D level lower in AIS group vs., controls (p=0.001); negatively correlated w Cobb angle (p<0.026) | Pts monitored for vitamin D sufficiency or deficiency |
| Gozdzialska et al (2016) | 50 premen (12.6) & 50 postmen (14.6) AIS girls vs 50 premen (11.9) & 50 postmen (13.6) controls | Polish | Premen Gp: 25[OH]D 23.5% lower in AIS pts (p<0.05) than controls Postmen Gp: 25[OH]D 36.2% lower in AIS pts (p<0.000) than controls |
Vit D may be associated with scoliosis |
| Silva et al. (2017) | 36 girls and 7 boys AIS (15.3) | Brazilian | 97% AIS patients: 25[OH]D <30ng/mL insufficiency and deficiency | No relationship between vitamin D and Cobb angle |
| Catan et al. (2020) | 32 AIS girls vs. 32 gender and age-matched controls (14.75) | Romanian | 25[OH]D level in AIS pts sig lowered than controls (p<0.0001) |
4.1.3. Body Mass Index (BMI)
Many studies have shown that the BMI of AIS patients is significantly less than that of age-matched healthy controls (Table 3) [12, 13, 15, 26, 28, 29]. Wang et al. (2012) reported that for AIS patients aged 15-17 years, with a mean Cobb angle of 30.4 degrees, 6.8% had BMI <5th percentile, whereas for the control group, only 3.4% had BMI <5th percentile [15]. Herschkovich et al. (2014) and Zheng et al. (2017) reported similar findings [30]. The prevalence of severe spinal deformities was 3-fold higher in the underweight group as compared with the overweight group [13].
4.1.4. Asymmetric Foot Biomechanics
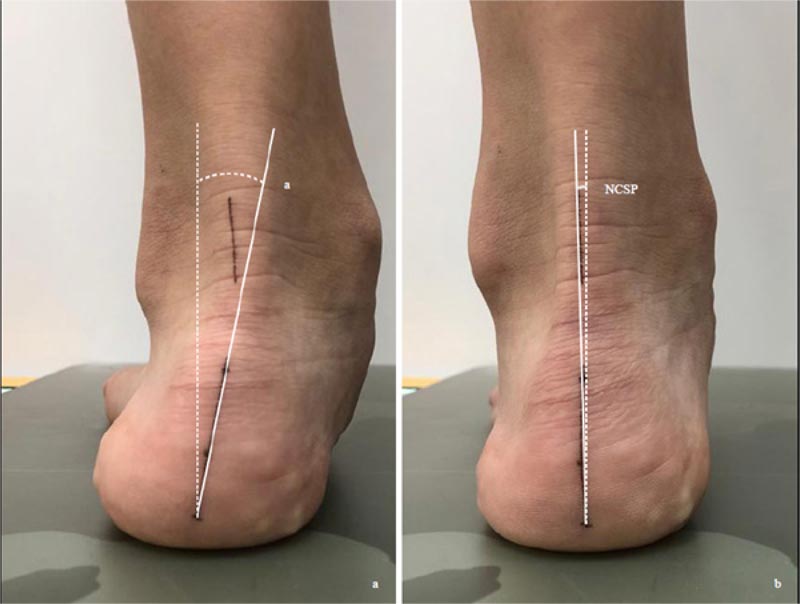
There is a lack of studies on the relationship between asymmetric foot biomechanics and spinal curvatures. Yet, Bialek et al. (2015) and Lehnert-Schroth (2007) both advocated examination and exercising of the foot in the management of AIS patients [31, 32]. Lee et al. (2018) evaluated 52 juvenile patients with a mean age of 79.5 months with excessive foot pronation and mild idiopathic scoliosis [16]. For those with the relaxed calcaneal stance phase angle difference (RCSPD) of >3 degrees between both feet, custom foot orthoses were prescribed. RCSP is the angle between the midline of the posterior calcaneus and the vertical line when the patient stands relaxed (Fig. 2). At 18 months post-intervention, in the group with the Cobb angle 10 degrees -19 degrees (n=20), the Cobb angle reduced from 17.29±1.8 degrees to 12.43±3.62 degrees. For the group with Cobb angle 20 degrees -24 degrees (n=9), the Cobb angle decreased from 21.62±1.54 degrees to 14.1±5.18 degrees. The improvement is most significant in patients < 6 years of age and a Cobb angle <25 degrees. The Cobb angle improved most at nine months post-intervention [16]. Improvement of Cobb angle, however, was not seen in patients with curvature ≥ 25 degrees. The mechanism involved is not understood; the asymmetric foot biomechanics may reduce the vertebral rotational instability, which often contributes to the progression of scoliosis [33].
| Studies | Sample | Age | Cobb | Height Estimation | Findings |
|---|---|---|---|---|---|
| Qui et al. 2008 (retrospective controlled study) |
613 AIS girls; vs. 449 healthy | 12-16 (Chinese) |
31o | Bjure's formula | Sig. lower in AIS girls |
| Wang et al. 2010 (Prospective controlled study) |
290 skeletally mature AIS girls vs. 80 healthy controls | 16-20 (Chinese) |
20-40o; >40o |
Arm span used, instead of body height | BMI sig lower in AIS girls with moderate and severe curves |
| Wang et al 2012 (Retrospective controlled study) |
332 AIS boys vs 356 age-matched controls | 10.5-19.5 (Chinese) |
30.4o | Bjure's formula | 15-17 age; BMI sig lower in AIS boys |
| Oh et al. 2014 (Prospective randomized controlled study) |
420 AIS pts vs. 409 healthy controls | 19 (Korean) | 23.2o | Bjure's formula | 10-25o curve: BMI sig. lower than controls |
| Herschkovich et al. 2014 (Retrospective study) |
59,039 male and 44,210 females | 17 (Israeli) |
Not reported | IS prevalence sig. higher in underweight males and females; | |
| Wang et al. 2016 (Prospective case-control study) |
47 AIS boys vs. 40 age and sex-matched healthy controls | 12-20 (Chinese) |
40-70o | Bjure's formula | Sig lower BMI in AIS vs. controls |
| Matusik et al. 2016 (Prospective study) | 204 girls, 55 boys, with IS | 14.21 (Polish) |
10-39o; >40 |
Bjure's formula | BMI sig. higher in pts w severe curves than those w moderate curves |
| Kim et al 2020 | 133M & 301F IS pts, vs. 8297M and 7681F | Korean | Not reported | BMI sig lower in IS pts (p<0.001) |
| Scoliometer (ATR) |
| X-ray if the ATR >5o |
| BMI (kgs/m2) |
| BMD (QUS) |
| Serum 25[OH]D |
| Foot Assessment |
4.2. Clinical Implications
4.2.1. Clinical Examination
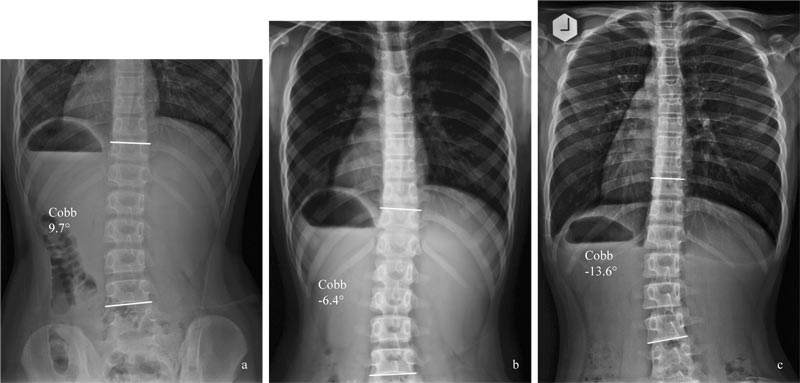
Generally, scoliosis screening is performed with a scoliometer, with a cut-off point between 5 degrees -7 degrees. It has to be noted that due to the presence of a rib cage, the angle of trunk rotation (ATR) is usually larger in a thoracic curve than in the thoracolumbar or lumbar curve, for a given curve magnitude. Depending on the outcome of the measurement, patients are either prescribed observation or be followed up by radiographic examination. When the Cobb angle is ≤ 20 degrees, observation is generally prescribed [1], and no further investigation will be performed.
Given the potential risk factors for curves progression, it is proposed that pre-menarcheal, skeletally immature patients with mild curvatures be also assessed for BMI [15], vitamin D level [10], and bone mineral density [14]. Also, the foot should be examined for bilateral differences in excessive pronation or supination (Table 4).
In assessing the BMI, the arm span of the patients can be used instead of the body height. Studies have shown that there is a high linear correlation between arm span and standing height in healthy children and adolescents (r2 =0.99) [34]. The BMI is calculated as body weight in kilograms divided by the square of arm span [34]. When BMI <5th percentile, it is regarded as low. Kim et al. (2020) recently reported that using the World Health Organization BMI z score cut-off would under-estimate the prevalence of AIS [19]. They instead proposed to use the cut-off point of <18.5kg/m2 as a criterion for defining mild underweight. The criterion may be used to screen early AIS cases [19].
Vitamin D levels can be assessed using blood samples and indirectly by quantitative ultrasonography (QUS) [35]. Yu et al. (2013) showed that QUS BMD could be an indicator of vitamin D status in young children [35].
Measurement of BMD may help identify patients with a higher risk of curves progression. QUS, which is devoid of radiation, may be used to evaluate the bone mineral status and bone fragility of growing children [36]. QUS measures the speed of sound (SOS) and broadband ultrasound attenuation (BUA) [37]. The speed of sound (SOS) measurements along the length of a long bone provide information not only on bone density but also on micro-architecture, cortical thickness, and bone elasticity [38, 39]. Readings from QUS cannot be used interchangeably with DEXA results [40], as they do not measure identical properties of bone tissues [37].
Du et al. (2015) compared the SOS of QUS of AIS girls with healthy age- and sex-matched controls [41], and found that AIS patients had lower bone quality than the controls. In the AIS patients, 20.5% had a z score of SOS ≤ 2, indicating low bone mineral content [41]. The difference was statistically significant for younger patients aged 10-14 (p =.000), but not for more skeletally mature patients aged 15 or above. Skeletally immature patients were more osteopenic [41]. The results supported the findings that QUS can be used as a screening tool to assess bone quality in children [41-44].
The feet should also be examined for asymmetric foot biomechanics. Lee et al. (2018) used a bilateral difference of RCSP of >3 degrees as a cut-off point. As accurate marking of the calcaneus is subjected to errors, we propose to use the difference between the RCSP and the neutral calcaneal stance phase (NCSP) instead (Fig. 2). NCSP is the angle subtended by the midline of the calcaneus and the vertical, with the subtalar joint in the neutral position. The difference between RCSP and NCSP represents the extent of excessive foot pronation or supination. A comparison of the difference bilaterally would reveal if asymmetric foot biomechanics is present.
4.2.2. Proposed Classification of Risk of Progression
4.2.2.1. In Skeletally Immature Patients with Mild Idiopathic Scoliosis
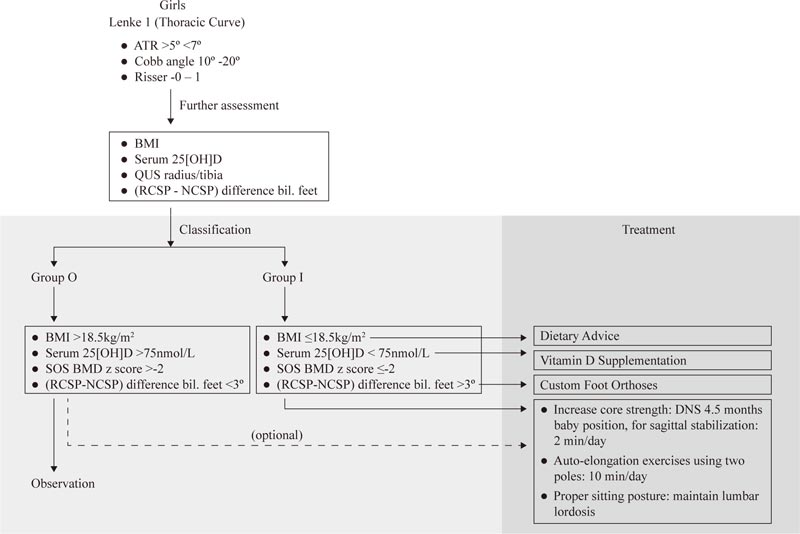
Given the identification of some prognostic factors in skeletally immature patients with mild curves, it may be time to re-think the observation strategy. It is suggested that pre-menarcheal, skeletally immature patients (< Risser 2) with mild curves be divided into two groups. The observation group (Group O) has a lower risk of progression, and the intervention group (Group I) has a higher risker of progression. Group O may include patients with BMI >18.5kg/m2, with normal serum vitamin D (>75 nmol/L), SOS BMD z score >-2, and (RCSP-NCSP) difference <3 degrees bilaterally. Group I may include female patients with a thoracic curve, BMI <18.5 kg/m2, serum 25[OH]D <75 nmol/L, SOS BMD z-score < -2, and (RCSP-NCSP) difference >3 degrees bilaterally (Table 4).
4.2.2.2. Proposed Intervention
We are unable to find any studies evaluating the effects of improving serum 25[OH]D level, BMD, and BMI on the scoliosis curvatures. Yet, we opined to modify these risk factors in Group I patients, despite SOSORT held that nutrition and foot orthoses do not play any role in the management of idiopathic scoliosis [1]. The BMI, BMD, and foot biomechanics may act within a narrow window in the development of idiopathic scoliosis. Improving those factors beyond the early stages of curvature development when scoliosis becomes structural may not be helpful.
Early modification of risk factors may modify the course of the disease. Karski et al. (2019) showed that abstinence from weight-bearing and vitamin D3 supplementation straighten the tibial varum in infants below three years of age [45]. Yet, after the age of 3, similar treatments have no outcome, and patients require surgery for correction. Tibial varum or medial compartment arthrosis in adults would not respond to vitamin D3 supplementation, even in the presence of vitamin D insufficiency or deficiency. Similarly, interventions that may be effective in mild curvatures in skeletally immature patients may not be useful when the curvatures become more advanced.
For patients with low BMI, dietary advice is suggested (Fig. 3). Those with serum 25[OH]D level <75 nmol/L should increase their dietary intake of vitamin D to increase the BMD [46]. Cheng et al. (2005) showed that increase cheese intake increases cortical bone mass accrual [47], suggesting that dietary manipulation can improve the BMD in children. For young patients with mild spinal curvatures and asymmetric foot biomechanics, custom foot orthoses may be prescribed.
| Parameters | Group O | Group I |
|---|---|---|
| Gender | Male | Female |
| Risser | ≥ 2 | < 1 |
| Serum 25[OH]D | ≥ 75nmol/L | < 75 nmol/L |
| Bone mineral density | SOS BMD z score >- 2 | SOS BMD z score ≤- 2 |
| Body mass index | ≥18.5 kg/m2 | < 18.5 kg/m2 |
| (RCSP-NCSP) | <3o | ≥3o |
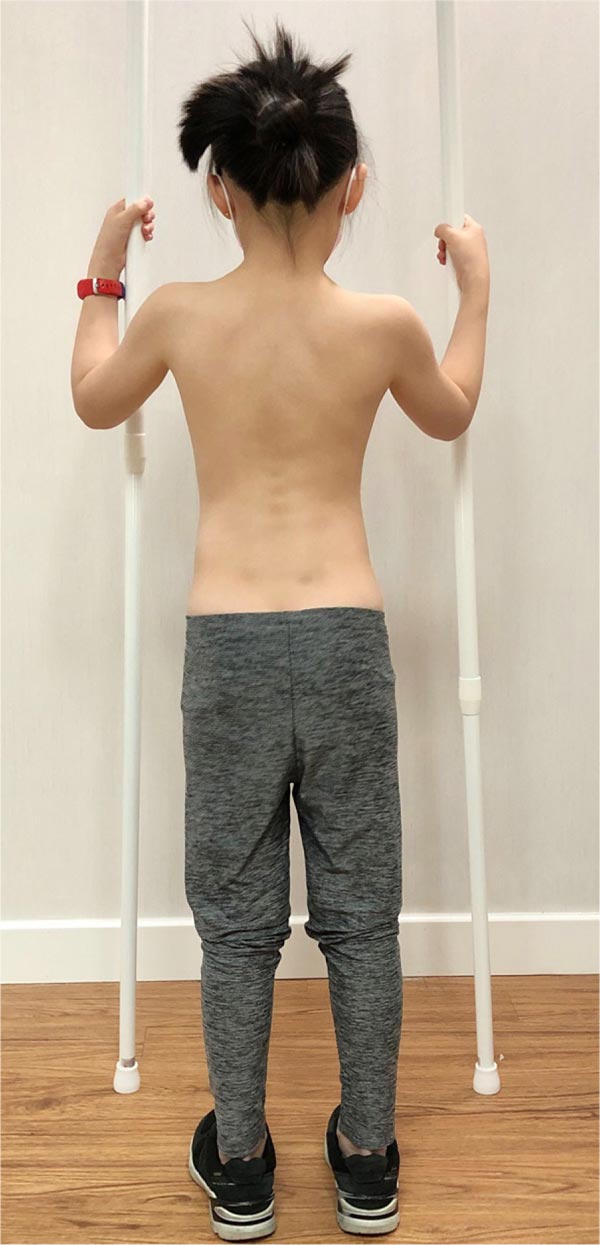
At-risk patients should learn simple PSSE exercises to stabilize the curvature. Simple dynamic neuromuscular stabilization (DNS) exercise, adopting a 4.5 months baby posture, may be prescribed for core strengthening [48]. The patient lies supine and flexes both hips and knees to 90 degrees, and uses the diaphragm for breathing (Fig. 4). This position is to be held for 1-2 minutes, and the exercise has to be performed 1-2 times daily. The exercise trains the brain and regulates the intra-abdominal pressure [48]. Concurrently, the child can perform the auto-elongation exercise using two poles (Fig. 5) [32].

Apart from simple exercises, the patient should adopt a proper sitting posture. Von Loon et al. (2009) have shown that forced lordosis in the thoracolumbar area would reduce the curve magnitude of a double major curve by >15% [49]. Whether the adoption of proper sitting posture would benefit other curve types has not been studied. Yet, the children should adopt corrective postures during daily activities (Fig. 6).
For Group I patients, active intervention is thus suggested (Fig. 7), particularly given the recent findings of a high progression rate (66%) in adolescents with curves 15 degrees – 19 degrees, and Risser 0-1. More importantly, perhaps, 7.8% of the patients progress to >45 degrees and only 3% of them have their curves resolved [4]. Lonstein and Carlson (1984) reported that resolution is more common in less mature patients with curves <15 degrees [50]. These justify the need for early intervention in Group I patients. Also, the intervention is free of side effects. A daily dose of 400-800 IU, when indicated, is safe, particularly in the presence of vitamin D insufficiency and deficiency [51]. Further, a proper made custom foot orthoses would not cause any complications.
Whether these interventions have any impact on mild scoliosis curvatures in young patients with Risser -0 to 1 remains to be studied. However, the management would possibly help prevent some future morbidities that are associated with low BMI, low 25[OH]D, low BMD, and asymmetric foot biomechanics. In this perspective, the intervention is justified and should not be regarded as over-treatment.
5. LIMITATION AND FUTURE STUDIES
The above discussion is based on existing knowledge. There is, at present, no evidence to support the classification of mild scoliosis based on the risk of progression, and the effectiveness of the intervention in the management of mild scoliosis curvatures.
Studies are required to determine if the classification of skeletally immature patients with mild curvature measuring < 20 degrees, and Risser -0 and 1 into Group O and Group I, is reliable and clinically meaningful. Also, the cut-off points for each of the progression risk factors need to be determined.
The effectiveness of the proposed intervention on scoliosis requires studies and validation, although the management may reduce future morbidities associated with low serum 25[OH]D, BMD, and low BMI.
CONCLUSION
Instead of passive observation, the current paper proposes active intervention in pre-menarcheal, skeletally immature patients with Risser < 2, Cobb angle ≤ 20 degrees, low BMI, 25[OH]D, BMD level, and with asymmetric foot biomechanics. Research is required to prove if the intervention is clinically useful or not in the management of skeletally immature patients with mild curvatures.
CONSENT FOR PUBLICATION
Informed consent was obtained from the patient for accompanying images.
FUNDING
No funding to declare.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
Declared none.


