All published articles of this journal are available on ScienceDirect.
LCH of the Scapula in a 2-Year-Old Masquerading as an ABC: A Case Report and Literature Review
Abstract
We describe a unique case of Langerhans Cell Histiocytosis (LCH) arising in the scapula of a 2-year old male child masquerading as an aneurysmal bone cyst (ABC) at clinical presentation and on imaging. Scapular involvement is only occasionally noted in LCH cases. Solitary bone involvement in our patient’s age group is uncommon in LCH without multi-organ involvement. Careful pathologic examination and immunohistochemistry was crucial in establishing this diagnosis due to the presence of a solitary lesion with fluid-fluid levels.
1. INTRODUCTION
A rare case of Langerhans Cell Histiocytosis (LCH) of the scapula in a 2-year-old male child who presented with a diffusely enlarged left scapula that was warm to touch with severe pain on movement is described in this study. LCH is an extremely rare histiocytic disorder with an incidence of 2 per million in children, affecting males to females in a 2:1 ratio [1, 2]. Our patient’s lesion was initially believed to be an aneurysmal bone cyst (ABC) based on clinical presentation and imaging appearance. ABCs are expansile, non-malignant bone lesions that predominantly affect pediatric populations. They frequently present with localized pain and swelling and upon plain radiography, they will typically appear as lytic expansile solitary lesions of the metaphysis. [3] The average age of presentation is 10.2 years and the most commonly affected sites are the femur, tibia, spine, humerus, and pelvis. [4] Our patient’s lesion was determined to be LCH with extensive hemorrhage and focal secondary ABC changes following pathological analysis [5, 6]. Comprehensive workup showed no other bone or organ involvement. There were only two reported cases in the literature of LCH masquerading as an ABC, with this being the third. Additionally, solitary bone involvement at this age and of the scapula is highly atypical in LCH. Hence, our patient is unique due to his lesion’s imaging features, clinical presentation, and location.
2. CASE PRESENTATION
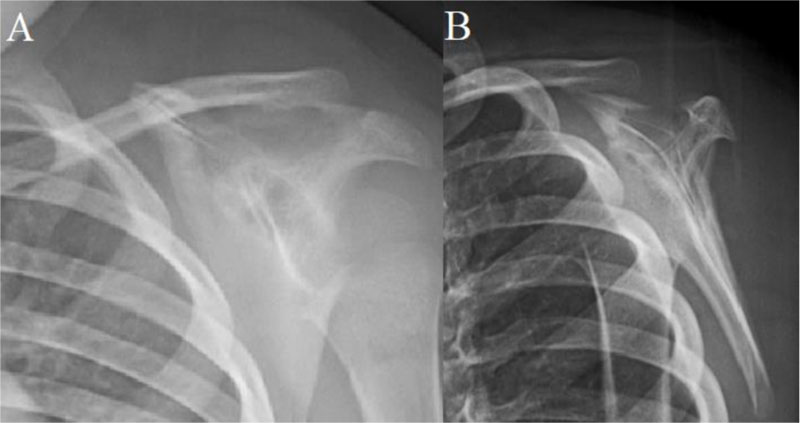
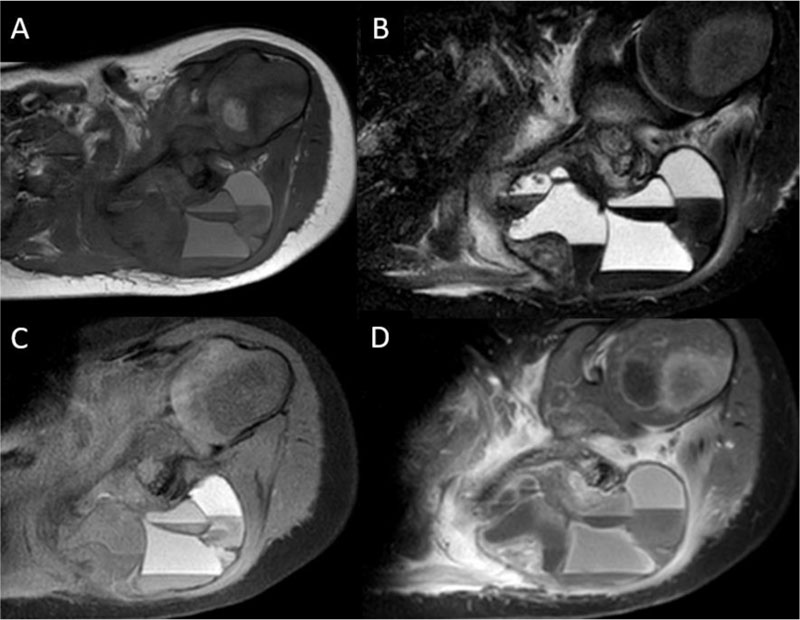
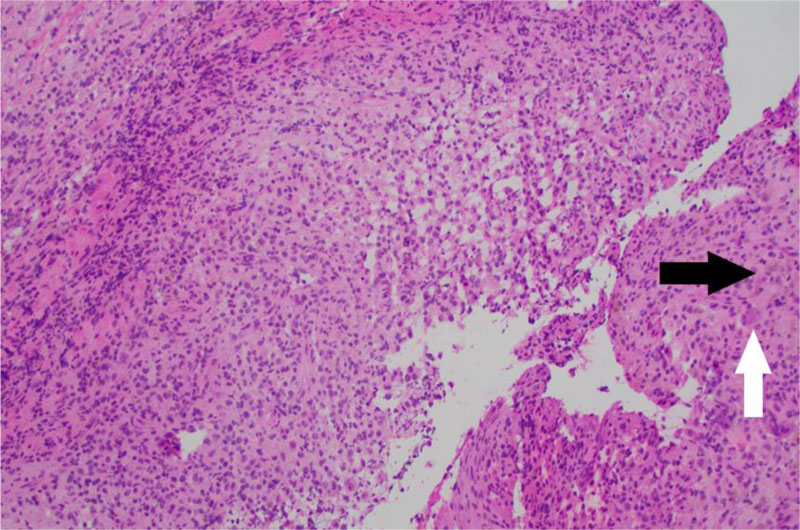
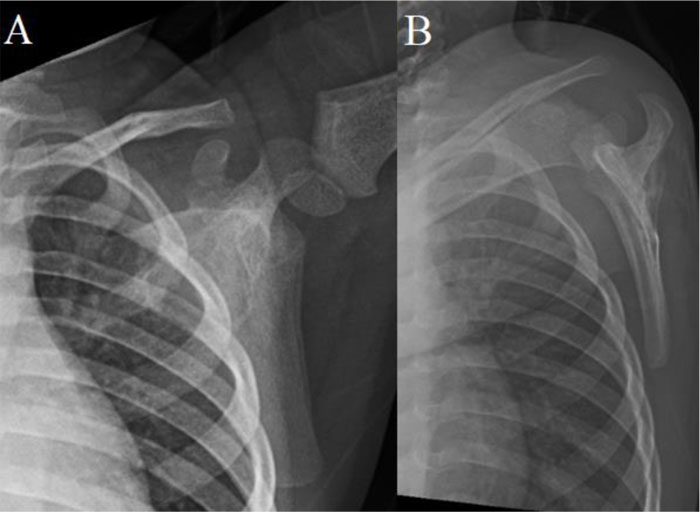
A 2-year-old male child with no significant medical history presented to the pediatrician with 1-month of left shoulder pain, forward positioning of the shoulder, and a protuberant scapula. There was no history of trauma, congenital defects, or other symptoms. Initial radiographs revealed a lytic, expansile, radiolucent scapular body bone lesion (Fig. 1). After orthopedic oncology consultation, coordination of MRI and open biopsy under a single episode of general anesthesia (GA) was planned [7]. MRI with and without Gadolinium revealed the presence of an expansile, multi-loculated, septated lesion involving the scapular body and spine (Fig. 2). Multiple fluid-fluid levels were noted within the lesion, reflecting intralesional blood products. No solid component was apparent on any MRI images. Perilesional intraosseous edema in the surrounding scapula and adjacent periscapular muscles was noted, suggesting either occult pathologic fracture or secondary ABC associated with primary chondroblastoma, LCH, osteoid osteoma/osteoblastoma, or infection [8]. Immediately following MRI, the patient was transported to the OR where an open biopsy was planned. Under general anesthesia, fluoroscopy was used to localize the bone lesion and plan the incision. A 3cm incision was made longitudinally over the body of the scapula extending cephalad to the scapular spine and dissection carried down to the bone. A scalpel blade was utilized to enter the almost non-existent outer cortex. A 1 cm diameter window was created for the biopsy. The tissue obtained consisted of multiple fragments of red-brown tissue. Frozen section favored ABC based on the presence of bloody and cystic areas associated with histiocytes, bland fibroblasts, osteoclast-like giant cells, and hemosiderin deposition (Fig. 3A, B). The incision was then extended and the entire lesion was completely curetted, leaving only a rim of bone around the native scapula. A high speed bur was used to further extend the margins around the lesion to ensure it was entirely removed. During intralesional curettage, the gross appearance was again felt to be consistent with ABC given the findings of blood-filled pools separated by septations. The resulting defect was filled with synthetic bone filler (Cerament, BoneSupport, Inc) and defect filling was confirmed fluoroscopically .
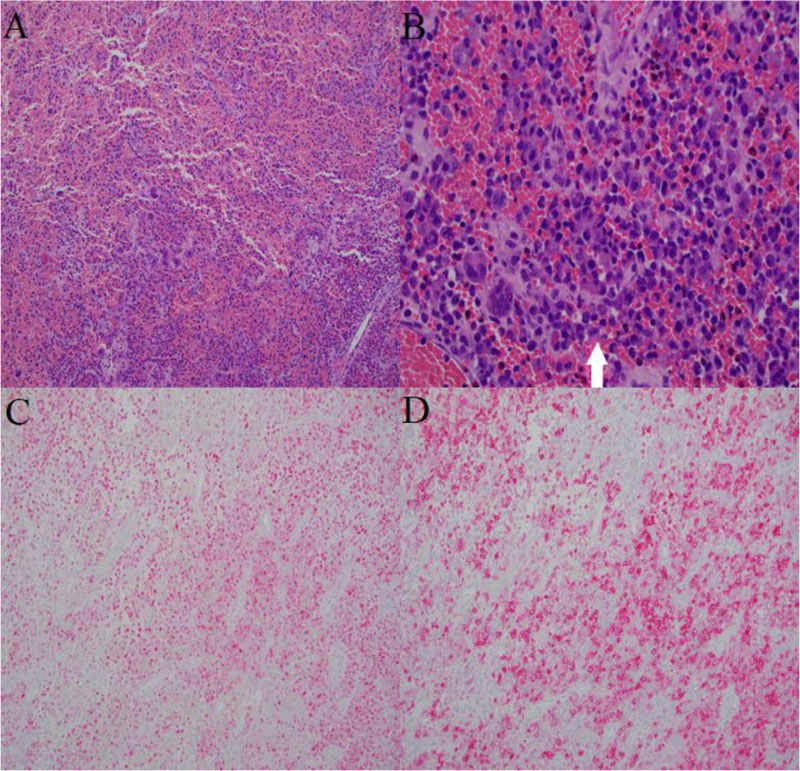
Final pathological analysis showed loose aggregates of histiocytes with nuclear grooving and eosinophilic cytoplasm admixed with osteoclast-like multinucleated giant cells, eosinophils, lymphocytes, and hemosiderin-laden macrophages set in an extensively bloody background (Fig. 4A, B). Blood-filled cystic spaces lined with fibroblasts, multinucleated giant cells, and focal collections of histiocytes with nuclear grooving were also focally identified. Histiocytes were positive for CD1a and S100 by immunohistochemistry, consistent with Langerhans cells (Fig. 4C, D) [9, 10]. Given those findings, the lesion was diagnosed as LCH with extensive hemorrhage and focal secondary ABC changes rather than primary ABC.
Subsequent evaluation by Pediatric Oncology included staging with skeletal survey and laboratory work. All imaging and laboratory analysis suggested no additional LCH. Observation was elected going forward. At three months post-operation, the patient had no pain, was participating unrestricted in all activities, and had regained full active range of shoulder motion without local tenderness or masses. Three-month scapular radiographs showed graft material incorporation without any recurrence (Fig. 5).
3. DISCUSSION
The differential diagnoses (DDX) for this patient shifted based upon each piece of clinical, imaging, and pathologic evidence obtained. Due to the patient’s age and progressively enlarging, painful intraosseous bone lesion, metastatic neuroblastoma, osteomyelitis, and LCH were considered in the initial DDX. [5, 11-15] Inclusion of neuroblastoma metastatic to the bone in our initial clinical-imaging differential diagnosis was due to the young age of the patient with painful aggressive bone involvement. Langerhans cell histiocytosis was considered initially due to the patient’s young age, although the scapula is the least common skeletal site. [16, 17] The MRI findings shifted the differential to primary ABC, secondary ABC, or telangiectatic osteosarcoma [18-20]. Imaging findings were inconsistent with metastatic neuroblastoma. In LCH, MRI often shows well-defined lesions with a high T2 and low T1-weighted appearance but only rarely fluid-fluid levels. [17] In this case, the radiographic appearance was atypical of LCH, particularly on MRI. The presence of perilesional edema on MRI, however, suggested the possibility of an associated pathologic fracture, as well as secondary ABC associated with chondroblastoma, LCH, osteoid osteoma/osteoblastoma, or infection. The intra-operative frozen section suggested primary ABC, as no malignant or primary component, and characteristic features of LCH were recognized. Only upon further review of the histology was LCH confirmed. The most distinctive radiographic findings, in this case, were fluid-fluid levels and septations. The most common causes for bone lesions with fluid-fluid levels are shown in Table 1, along with rare causes and a suggested mnemonic for these lesions (GOATS CSF) is as shown in Table 2. LCH is not included in either the suggested differential diagnosis or the mnemonic due to its extreme rarity.
Based on MRI, our patient was felt to most likely have an ABC, a benign bone lesion most commonly affecting children and presenting with localized pain, swelling, and even pathologic fracture. [21, 22]. Plain radiography typically reveals expansile radiolucencies circumscribed by a thin layer of periosteal bone, as evident in this case. On MRI, numerous blood-filled channels with fluid-fluid levels divided by septations were observed. [4, 23]. Both plain film and MRI findings in our patient suggested ABC, with the differential diagnosis, including secondary ABC and telangiectatic osteosarcoma. [24-26]. Histopathology of ABC shows cavernous blood-filled spaces without endothelial lining, benign giant cells, spindle cells, and thin strands of woven bone. [27].
| Aneurysmal Bone Cyst (ABC) | |
| Primary ABC | |
| Secondary ABC | |
| Giant cell tumors | |
| Chondroblastoma | |
| Osteoblastoma | |
| Simple Bone Cyst (after fracture) | |
| Telangiectactic Osteosarcoma | |
| Uncommon Causes | |
| Metastases (esp. Renal Cell and Lung primaries) | |
| Synovial sarcoma | |
| Synovial hemangioma | |
| Myositis ossificans | |
| Adamantinoma | |
| Neurogenic tumors | |
| G | Giant cell tumors |
| O | Osteoblastoma |
| A | Aneurysmal bone cyst |
| T | Telangiectactic osteosarcoma |
| S | Sarcomas (esp. synovial) |
| C | Chondroblastoma |
| S | Simple bone cyst and synovial hemangioma |
| F | Fibroxanthoma (non-ossifying fibroma) |
Only the final histology review provided a firm diagnosis of LCH. The change in diagnosis was based upon morphologic identification of the characteristic Langerhans cells confirmed by expression of CD1a and S100. In retrospect, the tissue fragments on the frozen section slides with the loose Langerhans cell histiocytic clusters admixed with osteoclast-like giant cells and hemosiderin deposition were originally misinterpreted at the time of the frozen section evaluation as being fibrous septae with bland fibroblasts and osteoclast-like giant cells similar to those seen in ABC. Both frozen section artifacts and the unusual presence of intra-tumoral blood-filled cystic spaces played a significant role in misinterpretation at the time of the frozen section evaluation. The presence of blood lakes within the tumor ultimately explained the fluid-fluid levels and septations noted on MRI.
The pathological cells of LCH are the Langerhans cells, which are characterized by their ovoid or shape, unobtrusive nucleoli, lobulated nuclei, and eosinophilic cytoplasm. [13]. The background stroma in LCH demonstrates eosinophils, neutrophils, and monocyte-derived histiocytes. Langerhans cells of LCH lack dendritic cell processes, unlike normal skin Langerhans cells. [13]. However, similar to skin Langerhans cells, the pathologic cells of LCH express the markers CD1a, S100, and CD207 (langerin). Diagnosis of LCH is ultimately based on pathology results, with fine-needle aspiration or core biopsy usually sufficient to yield the required material for diagnosis. [28, 29]. However, the identity of the Langerhans cells must be confirmed via positive immunostaining for CD1a, S100, or CD207 (langerin) or electron microscopy identification of Birbeck granules. [30-32]. Furthermore, over 50% of patients with LCH carry an oncogenic BRAF V600E mutation. [33, 34]. Our patient was not evaluated for BRAF mutation. The V600E mutation is not specific for LCH, as it is also found in melanoma, hairy cell leukemia, and Erdheim-Chester disease (another disorder of histiocytes). [33, 35-37].
Following diagnosis, imaging is required to stage the extent of involvement. A skeletal survey, skull films, and chest radiographs are often sufficient. [18] However, depending on suspected systemic involvement, CT, PET, and/or MRI may also be useful. [38-40] In our case, pediatric oncology did not recommend additional staging evaluation due to the lack of signs indicative of recurrent disease or multisystem disease. For isolated skeletal involvement, no additional treatment beyond surgery is necessary, although careful observation is prudent to ensure that additional lesions do not arise, particularly in young patients such as our case. [7, 41] While there is no universally accepted treatment protocol for LCH apart from cranial locations, minimally invasive and localized approaches are typically preferred. [28] Solitary bone lesions have a good prognosis with curettage with low rates of recurrence, particularly among pediatric patients. [42, 43] In patients with multifocal bone involvement, corticosteroids and vinblastine are typically used with good results and they are well tolerated. [7] Our patient was treated in Stratum VI (Natural History and Management) protocol in the LCH-IV International Collaborative Treatment Protocol for Children and Adolescents with Langerhans Cell Histiocytosis (www.cancer.gov › clinical trials › NCT02205762). [44]
CONCLUSION
Other reports of similar presentations are rare. Only two cases have been reported. One 8-year-old girl was discovered to have an occipital bump. MRI showed an expansile, destructive, and lytic mass located with multiple fluid-filled levels favoring an ABC as in our case. However, upon excision and biopsy, the characteristic features of LCH were found. [45] The second report involved a 2-year-old male patient with ABC secondary to LCH, and on the occiput. [46] Thus, the current report is the first documented instance of LCH masquerading as an ABC outside of the skull. In conclusion, we present an unusual case of LCH with extensive hemorrhage mimicking primary ABC on imaging modalities. Hemorrhagic LCH is an entity that should be considered in the differential diagnosis of bone lesions with fluid-fluid levels. Pathologic examination and a thorough immunohistochemical workup play a vital role in establishing this rare and challenging diagnosis.
LIST OF ABBREVIATIONS
| LCH | = Langerhans Cell Histiocytosis |
| ABC | = Aneurysmal Bone Cyst |
| GA | = General Anesthesia |
| DDX | = Differential Diagnosis |
CONSENT FOR PUBLICATION
We have obtained consent to publish from the patient.
STANDARD OF REPORTING
CARE guidelines have been used for conducting this research.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
None declared.


