All published articles of this journal are available on ScienceDirect.
Functional Outcomes After Salvage Procedures for Wrist Trauma and Arthritis (Four-Corner Fusion, Proximal Row Carpectomy, Total Wrist Arthroplasty, Total Wrist Fusion, Wrist Denervation): A Review of Literature
Abstract
Background:
Several salvage procedures for the arthritically destroyed wrist exist. Each of these has advantages as well as disadvantages.
Aims:
The aim of this article is to give practical insights for the clinician on: (1) biomechanical and clinical fundamentals of normal and impaired wrist motion; (2) difficulties in assessment of postoperative outcome between measured motion by the surgeon and self-reported outcome by the patient; (3) indications for each procedure; and (4) differences in functional outcome between partial and complete motion-preserving as well as complete motion-restricting salvage procedures.
Methods:
In trend, Proximal Row Carpectomy (PRC) is slightly superior over four-corner fusion (4CF) in terms of functional outcome, but the methodology-related postoperative motion is decreased for both procedures. Furthermore, PRC is easier to perform, needs lower costs, and has fewer complications than 4CF. Total Wrist Arthroplasty (TWA) has the advantage compared to PRC and 4CF that the preoperative motion values are preserved, but it is limited by decreased load-bearing capacity for the wrist. Total Wrist Fusion (TWF) is associated with a higher load-bearing capacity for the wrist than TWA, but it is limited for carrying out essential activities of daily living. Both PRC and 4CF can be combined primarily by wrist denervation. Wrist denervation alone does not impair the movement of the wrist.
Results and Conclusion:
Salvage procedures for the arthritically destroyed wrist should be detected regarding patients age- and gender-related claims in work and leisure. Not all of them can be successfully re-employed in their original occupations associated with high load-bearing conditions.
1. WHICH SALVAGE PROCEDURES EXIST AND WHICH WRIST MOTION IS NEEDED?
Salvage procedures for the treatment of painful post-traumatic or non-traumatic wrist Osteoarthritis (OA) contain generally five main groups: (1) Partial motion-preserving Partial Wrist Fusion (PWF) such as the four-corner fusion (4CF); (2) Partial motion-preserving resection arthroplasty such as Proximal Row Carpectomy (PRC); (3) Complete motion-preserving Total (or partial) Wrist Arthroplasty (TWA); (4) Complete motion-restricting Total Wrist Fusion (TWF); and (5) Partial or complete motion-preserving wrist denervation as an additional procedure to PWF or PRC, or as a sole procedure when the other procedures are not possible in special situations. It has been noted by Sterling Bunnel (1882-1957): “A painless stable wrist is the key to hand function“ [1]. That means that all motion-preserving salvage procedures at the wrist must ensure sufficient stability in order to provide functional tasks in activities of daily living for the patients. Fortunately, the overall wrist motion is not needed in every instance especially for the elderly or so-called low-demand patients. It is still widely known from the literature that for the wrist 5° to 40° of flexion, 30° to 40° of extension, and 10°/15° of radial/ulnar deviation or 40° of overall radial-ulnar motion arc are required only to perform the most essential activities of daily living, and 21 out of 24 of them are performed with the wrist mostly in extension [2-4], and noted that optimal wrist function in healthy subjects requires only a range of motion (ROM) from 10° of flexion to 35°of extension. Volz et al. [5] found that in normal volunteers the poorest performance was associated with only 15° of wrist extension. Recently, Biehl et al. [6] reported that elderly patients (34 wrists in 28 patients, average age 60.6 years) with severely destroyed rheumatoid wrists sustained radiocarpal fusions and rated their long-term outcomes with “satisfactory” in the presence of mean extension/flexion of 22.5° /15.97° whilst “unsatisfactory” with a mean extension /flexion of 11°/1.6°. Moreover, patients who sustained partial motion-preserving PRC rated their outcomes in Disability of Arm, Shoulder and Hand (DASH) questionnaire highly significantly better than patients sustained TWF (p < 0.001) [7], and these results are comparable to those after complete motion-preserving TWA utilizing the Patient-Rated Wist Evaluation (PRWE) [8]. However, all salvage procedures are not free of any difficulties and complications, thus, a detailed understanding of the risk factors is essential for surgeons so that patients may be counselled accordingly and that alternative treatment options may be considered [9].
2. WHERE DOES WRIST MOTION TAKE PLACE, AND WHAT CAN WE EXPECT WITH MOTION-PRESERVING PROCEDURES (EXCLUDING WRIST DENERVATION)?
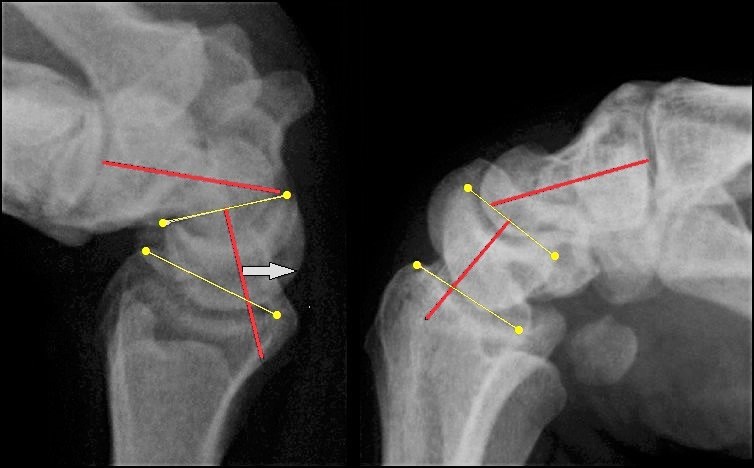
In the literature, it is still controversial about the relative contributions in radiocarpal and midcarpal joint during wrist motion (Fig. 1). The relative portion for flexion is reported to be 40 to 63% radiokarpal and 36 to 60% midcarpal, and for extension 50 to 66,5% radiokarpal and 33,5 to 50% midcarpal [5, 10-12]. For radial-ulnar deviation, the relative portion of midcarpal motion accounts approximately 75% vs. 25% radiocarpal [10, 13], and the centre of axis for this motion is placed up to 6,8 mm distal to the axis for extension-flexion at the proximal capitate pole [14, 15]. When the wrist is held in a neutral position, the relative portion of loading is 50% in radioscaphoid fossa, 35% in radiolunate fossa, and 15% in ulnocarpal fossa [16]. Wrist motion from extension-radial deviation to flexion-ulnar deviation (i.e. „dart-throwing“ motion) takes place in a midcarpal oblique axis which is angled 28° to 57° relative to the extension-flexion axis, whereas from extension-ulnar deviation to flexion-radial deviation (i.e. “reversed dart-throwing” motion) in radiocarpal joint [17, 18], however, Kane et al. [19] found that “dart-throwing” motion can also take place in radiocarpal joint. For circumduction, the largest total ROM in a human fresh-frozen cadaveric model is approximately 178° ± 10.5° and is oriented in the oblique direction of radial extension and ulnar flexion [20].
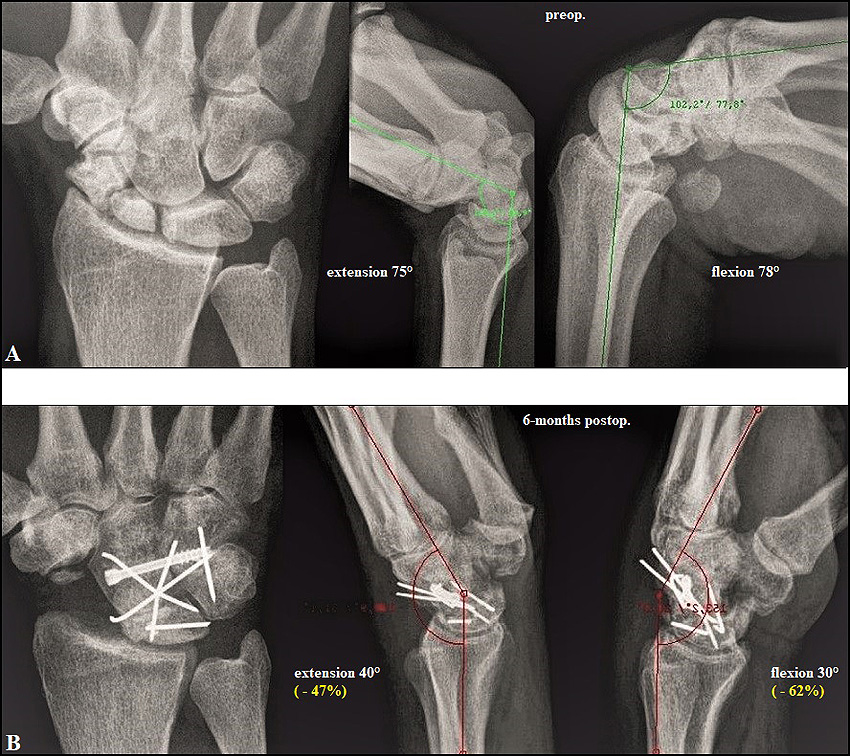
These biomechanical fundamentals declare that in every instance a PWF (i.e. radiocarpal or midcarpal) was done, extension and flexion are decreased approximately up to 50% compared to preoperative, despite preservation of carpal height (Figs. 2 A-B) [21]. Moreover, these biomechanics declare as well that after PWF the wrist is compromised due to the increased compressive forces in the surrounding intercarpal joints which is to be considered generally as a predisposition for secondary OA [22]. Furthermore, due to the coupled motion between extension-flexion and radial-ulnar deviation [23], impaired wrist extension-flexion inevitably can lead to impairment of “dart-throwing” motion [24], but it is not always observed after radiocarpal fusion, 4CF, and PRC [25].
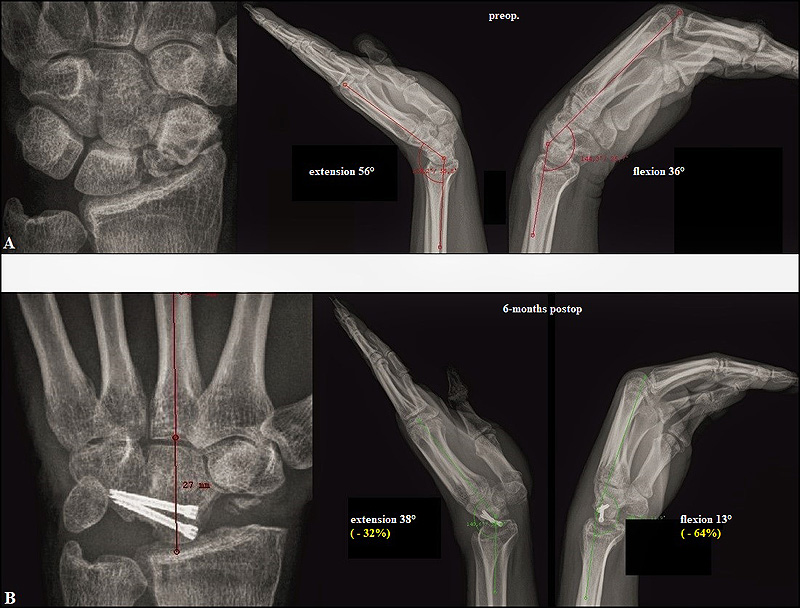
PRC (i.e. without replacement of midcarpal joint) is associated with loss of carpal height (i.e. translocation of the rotation centre to proximal) by creating “one new” radiocapitate joint in the absence of a distal congruent partner for the anatomically determined ellipsoid surface articulation like in a normal radiocarpal joint. Therefore, it also inevitably leads to a methodology-related decrease of wrist extension-flexion, but approximately 15% lesser than after 4CF (Figs. 3 A-B) [26]. In a human fresh-frozen cadaveric model with 3D computed tomography evaluation, it was found that despite wrist motion significantly decreases 28% for flexion and 30% for extension, the much more smaller contact area of the proximal capitate pole as compared to the articular surface of the lunate allows a greater action radius of the capitate in the lunate fossa accompanied by its translocation in all directions during motion, and resulting in an increase for flexion/extension to 140%/146% compared with in midcarpal joint of an intact wrist, and to 136%/135% compared with in radiocarpal joint of an intact wrist [27]. However, this unphysiological movement, combined with 3.8 times increase of contact pressure to a normal radiocarpal joint [28], makes this “new joint” susceptible to secondary OA as well.
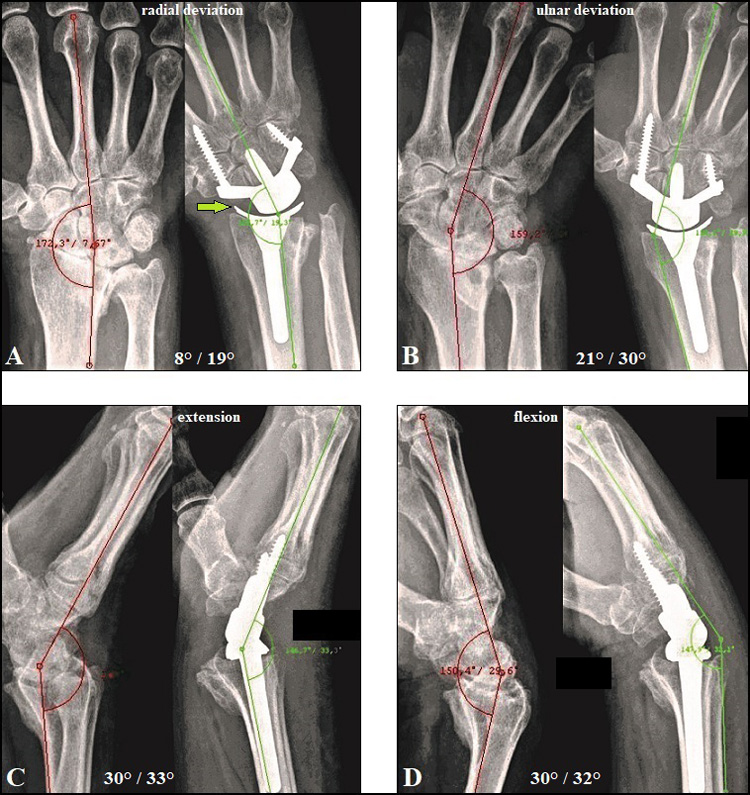
TWA utilizing the modern biaxial-anatomic 3rd generation types (Universal2/Freedom, Re-MotionTM, MaestroTM), introduced in the beginning of 2000, is also associated with removal of midcarpal joint, but it preserves both carpal height and the rotation centre, and maintains ellipsoid surface articulation (metal-on-polyethylene articulation, carpal peg into the capitate) like an intact radiocarpal joint. Thus, overall wrist motion (ROM) is slightly or significantly increased or at least unchanged postoperatively (Figs. 4 A-D [29, 30 ]), however, TWA is unable to completely restore circumduction (20% of a normal wrist is maintained with the Universal 2) as well as stress distribution like in a normal wrist [31-35]. A revival of the 2nd generation types (ball joint with metal-on-metal articulation, carpal peg into the 3rd metacarpal crossing the 3rd carpometacarpal joint) is seen with the Motec® [36].
3. WHAT IS EVIDENT WHEN WRIST MOTION IS IMPAIRED?
Adams et al. [37] demonstrated that, in young healthy subjects, limited wrist motion inevitably led to a statistically significant worsening of their ratings in activities of daily living (DASH, PRWE), and limited or completely restricted wrist motion with or without pain is subsequently accompanied by impaired power and performance both in elbow and shoulder [6, 38]. In patients sustained TWF, the lowest scores found were for perineal hygiene, using a screwdriver, and trouble using the hand in tight spaces such as changing spark plugs on the family car, followed by writing, drinking from a glass, turning a doorknob, combing hair, and using a hammer (i.e. “dart-throwing” motion) [39]. However, limited wrist motion can be compensated over a not clearly known time by increased activities of trunk and shoulder muscles such as the upper trapezius and deltoideus [37, 40, 41]. But the question is: are elderly patients with their age-related overall muscle degenerations able for this compensation mechanism, and if yes, how long, and what about the number of subsequently following functional disorders in elbow and shoulder in patients with longstanding impaired wrist motion?
4. DOES THE OBJECTIVE FUNCTIONAL OUTCOME MEASURED BY THE SURGEON ALWAYS CORRELATE WITH THE SELF-REPORTED OUTCOME BY THE PATIENT?
One question is not clearly answered currently: What is better in the assessment of postoperative outcomes at the wrist: measurement of movement by the surgeon or self-reported outcome by the patient? Despite some limitations, since introducing the DASH questionnaire by Hudak et al. [42] with the mean value of 10.1 (SD 14.88) in a normal population [43], this questionnaire has proven to be one of the most popular and reliable self-reported outcome evaluations for the wrist and hand all over the world, and McCullough et al. [33] found no significant differences in comparison to PRWE in assessment of self-reported outcomes after TWA. However, in the literature, it is still controversial whether postoperative objective measurements of wrist motion correlates with the self-reported DASH by the patients [44, 45], and care must be taken when pre-existent or concomitant disorders at the shoulder, elbow, forearm, and lower extremity are present [6, 46-48]. Yang et al. [49] found in patients sustained distal radius fractures that wrist extension, active thumb opposition, and the ability to make a full composite grip were among the strongest ROM measures associated with the QuickDASH whilst wrist radial deviation and forearm pronation were not statistically significantly associated with this score.
Furthermore, when evaluating the DASH or QuickDASH among patients between different studies, particularly in the elderly, newer studies revealed that age- and gender-related features should be borne in mind. Aasheim and Finsen [50] found that the mean DASH in women originates from 5 (aged 20-29 years) to 22 (aged 70-79 years) and to 36 (aged over 80 years) whilst in men with similar age groups from 5 to 13 and to 22, and they stated that the QuickDASH should be preferred because it gives the same information, and it is shorter and completed more often. On the other hand, Finsen [51] reported for the QuickDASH that socioeconomic factors with female predominance can lead to bias as well, the mean scores for women were 30 for those with the shortest and 9 with the longest education (p < 0.001) whilst for men 19 and 7 (p < 0.001). Poorer self-reported outcomes after wrist injury or surgery related to female predominance were also observed in terms of catastrophic thinking [52], appearance of complex regional pain syndrome [53-55], and women complain wrist malpractice four times more often than men (p < 0.005) and receive five times more frequently financial compensation than men (p < 0.001, mainly based on the causes “operative treatment should have been performed” and “wrong operative method applied”) [56].
5. COMPARING FUNCTIONAL OUTCOMES OF SALVAGE PROCEDURES AT THE WRIST
5.1. 4CF vs. PRC
Both procedures are indicated in Scapholunate Advanced Collapse (SLAC) and Scaphoid Nonunion Advanced Collapse (SNAC) when radioscaphoid joint alone is involved in arthritic changes (stage II), and 4CF as well when the midcarpal joint is involved additionally (stage III). Scaphoid fracture and scapholunate ligament disruption are mostly injuries of the young or younger active adults with male predominance. If scaphoid fracture is primarily undiagnosed or insufficiently treated, non-union occurs within averaged 8.2 years accompanied by first mostly asymptomatic arthritic changes at the radial styloid (SNAC I) radiographically, followed by symptomatic SNAC II within 17.0 years and SNAC III within 20 years, and SNAC IV (i.e. pancarpal OA, additionally involving radiolunate joint) within 31.6 years [57, 58]. The only one difference to SLAC is that OA here starts between the proximal scaphoid pole and radioscaphoid fossa in stage I, and not at the radial styloid as in SNAC. Therefore, 4CF and PRC are mostly performed in patients with mean age ranging from 42 to 56 years (range 8 to 84 years), and approximately 75% of them are males (range 53 to 96%) [59, 60]. Note that PRC is also an early surgical option for treatment of severe carpal trauma provided, in order to avoid ulnar carpal translocation after surgery, that the volar extrinsic radioscaphocapitate ligament, which is one of the most important stabilizer for the wrist, is not injured [61, 62]. Ulnar carpal translocation is also known as a rare wrist injury, often initially un- or misdiagnosed due to the unfamiliarity by treating physicians, and, despite primary or secondary ligamentous reconstruction, potentially leading to post-traumatic OA in the majority of all cases within 6.5 years [63-67].
Generally, PRC reveals better self-reported outcomes by the patients, has better functional outcomes, has significantly fewer complications than 4CF such presented as non-union, symptomatic hardware, and hardware failures, and management of non-union after 4CF is challenging [68-72]. Singh et al. [26] demostrated that the ROM for flexion-extension was 65% of the non-surgical side after PRC vs. 50% only after 4CF, and PRC shows a circumduction curve concentric with the non-surgical wrist. Saltzman et al. [60] published a systematic review involving seven studies (Levels I-III, 240 patients, 242 wrists) and found significant values after 4CF vs. PRC: extension 39° vs. 43°, flexion 32° vs. 36°, flexion-extension arc 62° vs. 75°, radial deviation 14° vs. 10°, grip strength compared to contralateral 74% vs. 67%, and an overall complication rate of 29% vs. 14%. However, 4CF is not always superior compared to PRC in terms of grip strength despite its significant loss of carpal height [73], and the shape of the proximal capitate pole does not influence the outcome after PRC [74].
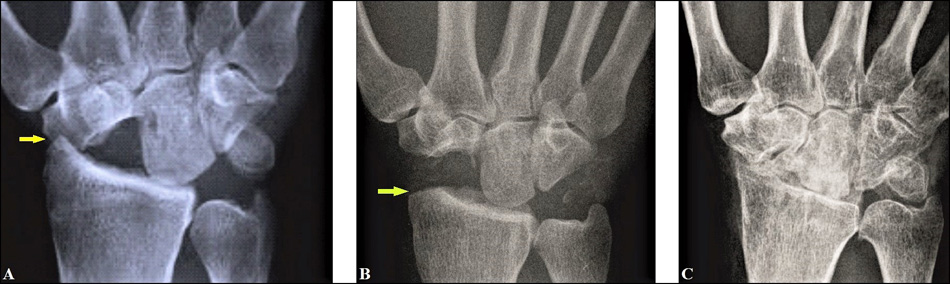
When comparing pre- and postoperative outcome studies, Mulford et al. [59] found the following values for 4CF vs. PRC: (1) flexion-extension motion arc worsened for both procedures from 79°/81° to 76° (both) associated with worsening of flexion (i.e. in opposite direction of surgical incision) from 39° (both) to 35° (4CF) and 36° (PRC) and extension from 39° to 35° after 4CF whereas extension improved from 38° to 42° after PRC, and (2) ulnar deviation improved for both procedures from 19° to 22° after PRC and from 16° to 17° after 4CF whereas radial deviation worsened for both procedures from 14° to 12° after 4CF and from 13° to 9° after PRC. However, not all of the patients sustaining PRC can be re-employed in their original occupations with heavy manual load [75, 76]. One main problem after PRC is the appearance of painful impingement between radial styloid and trapezium (Fig. 5 A) which can be avoided by radial styloidectomy [27]. In order to avoid ulnar carpal translocation, radial styloidectomy should be done radial-distal to the origin of the radioscaphocapitate ligament (i.e. level A) (Fig. 5 B) [77, 78]. Furthermore, PRC is more susceptible for the development of post-traumatic OA than 4CF (Fig. 5 C), but not all of these are clinically of relevance [59, 79, 80].
In conclusion, when considering both disadvantages and complications with a large number of 3,388 eligible patients, the conversion rate to TWF is significantly higher with 19.2% after PWF than 4.9% after PRC, and the authors of this study [81] stated that there may be a paradigm shift in the current practice patterns for salvage treatment of wrist arthrosis, more often considering PRC for all age groups. Noted that TWA is also a salvage option after failed 4CF or PRC [9, 82-84]. Last but not the least, the total costs are 425% greater for 4CF than PRC, implant costs for 4CF alone are 130% greater than the entire surgical encounter for PRC, and costs for plates and staples for 4CF are 70% and 240% greater relative to screws [85].
5.2. TWA vs. TWF, AND WRIST DENERVATION
Despite patients receiving TWA report difficulties with “writing”, “picking up small common objects”, “stacking checkers”, “lifting large light objects”, and take twice the time required to complete activities of daily living compared to a normal volunteer [33, 35], there is evidence in the literature that most of them are satisfied with their maintained or improved function (Figs. 4 A-D) associated with a significantly improved pain relief [32, 86]. Moreover, Ekroth et al. [87] reported that patients with failed TWAs utilizing older generation types would have a TWA again despite their long-term results being poor and many of them being revised to a TWF.
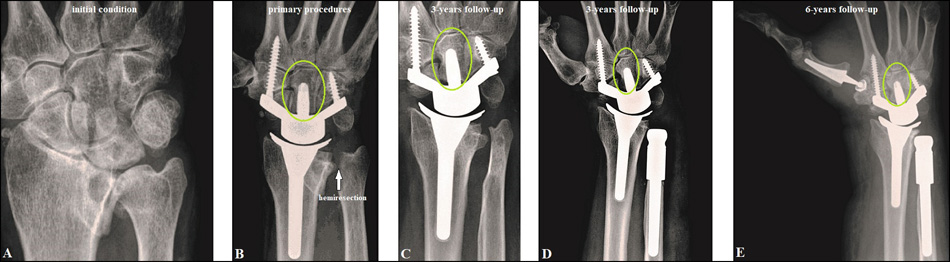
Recently, rheumatoid arthritis remains the most common indication with a relative portion ranging from 51 to 71% of all patients receiving TWA [86, 88]. From 1,213 patients receiving TWA in the USA from 2001 to 2013 (National Inpatient Sample Database, averaged 100 TWAs per year), 71% were females, and 75% of all patients were aged ranging from 50 to 79 years [89]. However, for rheumatoid arthritis the total number of wrist arthroplasties (in total 1,109 procedures in 1,069 patients including TWA and TWF, 83% females, age not available) in the UK has continuously and significantly decreased from 1996 to 2009 by approximately 50% which can be attributed by the effectiveness of the newer antirheumatic drugs [90]. TWA also has proven to be useful as a motion-preserving alternative to TWF for the treatment of post-traumatic wrist OA, primary wrist OA (Figs. 4 A-D and 6 A-E), gout as well as Kienböck’s disease, and resulted in a significantly better outcome than in patients who underwent a primary TWF [8, 91-95]. TWA for the treatment of post-traumatic wrist OA (including SNAC/SLAC) has reported to be a relative portion of 14% [86]. TWA is also an option for primary (or early) treatment of highly comminuted distal radius fracture in selected older and elderly patients [96, 97]. In the literature, no evident data exist regarding patients receiving TWA can load their wrists; Weiss and Akelman [98] advised their eligible patients not to load greater than 10 pounds which contains the implant from a safety perspective. However, there is a trend in literature toward younger patients with good bone stock [67, 84, 88].
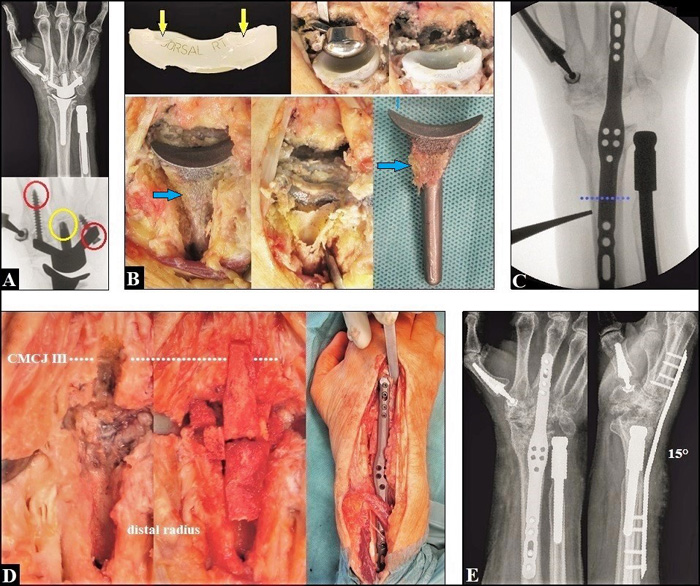
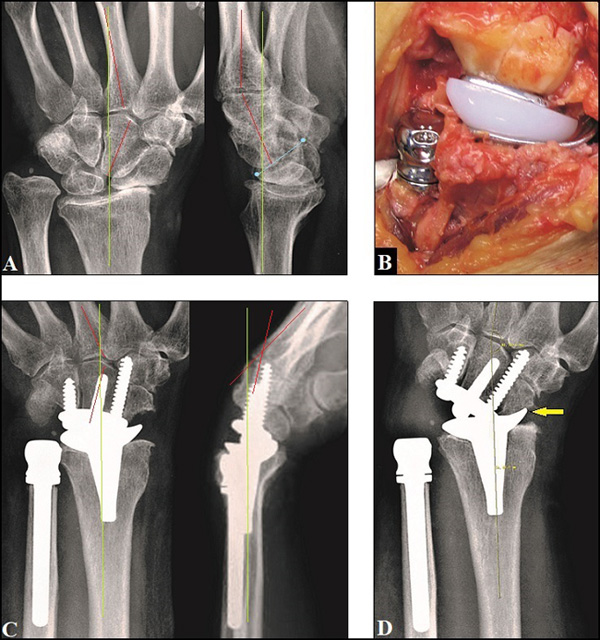
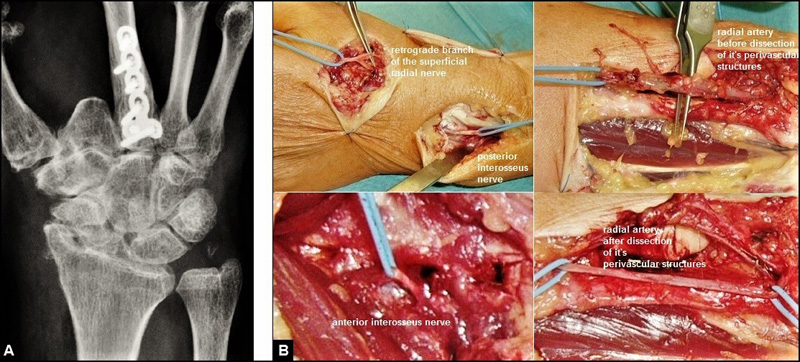
Recent evidence suggests that the complication rate of all 3rd generation TWAs is significantly lower than older generation types (p=0.002, range 0.1-2.9% vs.0.2-8.1%) [99], and its complication rate with 7% is slightly lower as well to those with 10% in patients undergoing a TWF, and TWF is associated with a higher percentage of perioperative device-related complications (6 vs. 3%, p < 0.001) and respiratory complications (0.54 vs. 0%, p < 0.05) potentially leading to higher costs of hospitalization than TWA in 2010 [88]. Recently, implant survival with the new 3rd generation TWAs is reported to be 90-100% at five years in most series (Fig. 6 A-E), but it declines from five to eight years [86]. Mid- to long-term survivorship is 78% for the Universal2, 86% for the Motec, 90% for the Re-Motion, and 95% for the Maestro [99], and these results are absolutely comparable with those after partial or total replacements at the shoulder, elbow and ankle which are much less debated in the literature than TWA [100-104]. However, the main problem is an unchanged loosening of its carpal components primarily based on mechanical imbalance and secondarily followed by polyethylene and/or metal wear (Figs. 7 A-B) [105, 106]. Risk factor for appearance of mechanical imbalance is when the carpal component is not correctly aligned in line of the 3rd metacarpal-capitate axis which is the central pillar for load transfer through the wrist, such observed in rheumatoid arthritis with progressive ulnar deviation in carpometacarpal joints (i.e. natural course of rheumatoid arthritis) [106], post-traumatic carpal and/or carpometacarpal malalignment (Figs. 8 A-D) [107, 108], or iatrogenic (Fig. 9 A-C) [109].

| Function | Universal 2 | Re-Motion | Maestro |
|---|---|---|---|
| (Charts pre- to postop., mean (range mean)) | (N=75 ext./flex., N=56 ud/rd) | (N=176 ext./flex., N=153 ud/rd) | (N=83) (2018 withdrawn by the company) |
| Extension° | +7,6 (-7,1 to +21) | +5,8 (-1 to +15) | +16 (+7 to +25) |
| Flexion° | +0,35 (-15 to +9,9) | -4,1 (-10 to +3,2) | -6 (-10 to -2) |
| Ulnar deviation° (ud) | +3,5 (-0,5 to +9) | +4,1 (-3,8 to +10) | +11 (+2 to +20) |
| Radial deviation° (rd) | -4,1 (-5,3 to -2,1) | -1,8 (-4,4 to 0) | +5,5 (+5 to +6) |
Comparing the functional outcome of the 3rd generation TWAs (Universal2, Re-Motion, Maestro), three trends can be observed (Table 1) [82, 92, 110-114]: (1) extension and ulnar deviation improved with all types but the Maestro is significantly superior, (2) radial deviation worsened with the Universal2 and the Re-Motion whilst significantly improved with the Maestro, and (3) flexion is equal or worsened for all types compared to preoperative. Impaired radial deviation with the Re-Motion, potentially leading to painful radial impingement despite partial removal of the scaphoid (i.e. radial-side diagonal resection), appears to be an implant-related issue (Fig. 8 D) [94], and it can only be avoided by the removal of the entire scaphoid [115]. This is similar to PRC before and after radial styloidectomy (Figs. 5 A-B), and reduced carpal height seems to be responsible for that appearance with the Re-Motion as well. In contrast, painful radial impingement is not observed with the Maestro (Fig. 4 A), and it may be justified by preserving resection-related carpal height due to its three various carpal heads combined with it in contrast to the Re-Motion (two carpal heads only, straight design of carpal plate) concave to distal shaped design of carpal plate. Equal or impaired flexion with all types (i.e. in opposite direction of the surgical incision) seems not to be an implant-related but a surgery-related issue. Loss of flexion (i.e. in opposite direction of surgical incision) more than for extension is also observed after 4CF and PRC Figs. (4 A-B and 3 A-B) [59] as well as in opposite direction of surgical incision for extension after volar plating for the treatment of distal radius fractures [116] and for flexion after surgical excision of dorsal wrist ganglions [117]. Hence, scar formation around the surgical incision accompanied by loss of elasticity of joint capsule is to be considered generally as a possible predisposition for impaired motion in the opposite direction after surgery.
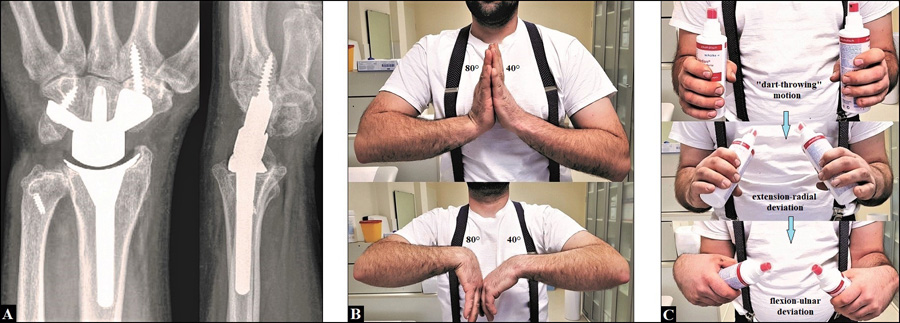
In conclusion, the Maestro is slightly superior both in terms of survivorship and functional outcome compared to the other 3rd generation TWAs. Despite these findings, the Maestro was withdrawn from the marketplace by the company in 2018, and surgeons have explanation misery to their patients when motion-preserving revision TWA is wished by the patient but not possible anymore by the surgeon (Figs. 7 A-E) [118, 119]. Note that the Maestro provides preservation of the "dart-throwing" motion arc and wrist circumduction in single cases (Figs. (10 A-C), video - attached as supplementary material).
TWF is unchanged a method of choice for primary treatment of traumatic and non-traumatic wrist OA especially for young and younger patients with high claims in their work and leisure [48, 120] as well as a reliable salvage option after a failed 4CF, PRC, and TWA (Fig. 7 A-E) [81, 121]. However, patients are limited in performing their activities of daily living. Adey et al. [122] reported that 20 of 22 patients sustained TWF for treatment of post-traumatic wrist OA would elect to have a procedure that could make their wrist move again if it were available, and 63.6% of them complained wrist pain, including severe pain in four patients. Moreover, when the wrist is fusioned then the extrinsic-related modulation of intrinsic long finger function is lost as well, hence, grip strength is decreased in every instance compared to a hand with a non-fusioned wrist [122, 123]. In the literature, it is still controversial about positions in which the wrist should be fusioned. Wagner et al. [48] reported satisfactory results for bilateral fusions with a mean of 13° ± 9° extension (range from 5° flexion to 30° extension), and a mean difference for right and left of 5° (range 0 to 15°). It has been observed that 30 to 53.6% of patients sustained TWF cannot be successfully re-employed with full use activities in their original occupations [124-126]. The main problem of TWF is persistent mircomotion in the 3rd carpometacarpal joint. Hardware failure (screw or plate loosening or fractures) with 16% is the most common complication after TWF, and 65% of these occurred when the fusion of the 3rd carpometacarpal joint was not additionally done [127]. Hence, the additional fusion of this joint should be recommended in every instance (Figs. 7 C-E).
Wrist denervation can be combined primarily with PWF/PRC or osteosynthesis of a complex injured wrist [66], and is to be considered as a last and sole option in order to reduce pain when both TWA and TWF are technically possible but the patient is unable for these procedures due to particularities in its private and social environment (Figs. 11 A-B) [118]. Furthermore, the simple and fast denervation of the wrist prior to TWA or TWF in young patients with post-traumatic wrist OA is a suitable and reliable option, does not decrease ROM, has no age limit, preserves grip strength, and still allows other procedures to be performed in the future [67]. Long-term results have been recognized satisfactory pain relief in up to 73% of cases [128-130], and pain relief was stable over time in 89% of cases at a mean follow-up period of 77 months [131]. Noted that patients without advanced stage of wrist OA benefit more from denervation than patients with wrist OA [132], but the majority of young patients with high load-bearing occupations cannot be successfully re-employed [133].
LIST OF ABBREVIATIONS
| OA | = Osteoarthritis |
| PWF | = Partial Wrist Fusion |
| 4CF | = Four-Corner Fusion |
| PRC | = Proximal Row Carpectomy |
| TWA | = Total Wrist Arthroplasty |
| TWF | = Total Wrist Fusion |
| ROM | = Range of Motion |
| DASH | = Disability of Arm, Shoulder and Hand |
| PRWE | = Patient-Rated Wrist Evaluation |
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
CONFLICT OF INTEREST
The author declares no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
The author would like to thank Dr. Stefan Richard Schiffhauer and Dr. Tino Beylich (both from Hospital Bad Salzungen GmbH, Germany) for their excellent support.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publishers Website along with the published article.


