All published articles of this journal are available on ScienceDirect.
The Effect of Air Tourniquet on Interleukin-6 Levels in Total Knee Arthroplasty
Abstract
Background:
Air tourniquet-induced skeletal muscle injury increases the concentrations of some cytokines such as interleukin-6 (IL-6) in plasma. However, the effect of an air tourniquet on the IL-6 concentrations after total knee arthroplasty (TKA) is unclear. We therefore investigated the impact of tourniquet-induced ischemia and reperfusion injury in TKA using the IL-6 level as an index.
Methods:
Ten patients with primary knee osteoarthrosis who underwent unilateral TKA without an air tourniquet were recruited (Non-tourniquet group). We also selected 10 age- and sex-matched control patients who underwent unilateral TKA with an air tourniquet (Tourniquet group). Venous blood samples were obtained at 3 points; before surgery, 24 h after surgery, and 7 days after surgery.
The following factors were compared between the two groups; IL-6, C-reactive protein (CRP), creatine phosphokinase (CPK), the mean white blood cell (WBC) counts, and the maximum daily body temperatures.
Results:
The IL-6 level at 24 h after surgery was significantly higher than that at any other point (p<0.01). No significant differences were observed in the WBC count, the body temperature, or the CRP, CPK, or IL-6 levels of the two groups at any of the time points.
Conclusion:
The effect of ischemia and reperfusion due to the use of an air tourniquet on increasing the IL-6 level was much smaller than that induced by surgical stress in TKA.
INTRODUCTION
Surgical stress can initiate various reactions. The following responses have been reported: increased circulating concentrations of cortisol and catecholamines (stress hormones), the synthesis and release of proinflammatory cytokines, and the induction of the synthesis and release of C-reactive protein (CRP) as an acute-phase protein, as well as metabolic change, which include the possible induction of lipolysis or hyperglycemia [1]. Surgical stress has been evaluated based on the body temperature, white blood cell (WBC) count, CRP, and interleukin-6 (IL-6) levels as stress markers [2-5]. To evaluate surgical stress, were also measured the levels of interleukin-1β, inerleukin-8, interleukin-10, tumor necrosis factor, and cortisol [6, 7] as well as the degree of pain after surgery (in cases of minimally invasive surgery) [8]. It is now recognized that IL-6 is a major mediator of the acute-phase protein response after many kinds of surgery [1, 9, 10].
Similarly, studies in the orthopaedic field have investigated IL-6 as a surgical stress indicator for several kinds of operations [11-14]. The IL-6 level at 24 h after unilateral total knee arthroplasty (TKA) was reported to range from 400-535 pg/dl [1, 11, 12], and that at 24h after unilateral total hip arthroplasty (THA) was reported to range from 62-162 pg/dl [12, 15, 16]. Furthermore, the IL-6 level at 24 h after bilateral TKA was reported to reach 1098 pg/dl when an air tourniquet was used [11]. However, why the IL-6 level is higher after TKA than after THA is unclear.
Huda et al. [17] found that tourniquet- induced skeletal muscle injury increased the plasma concentrations of some cytokines, including IL-6. We hypothesized that this might be caused by tourniquet-induced ischemia and reperfusion injury. However, there are few reports concerning the effect of tourniquets on IL-6 levels after TKA [18].
Thus, in the present study, the impact of air tourniquet-induced ischemia and reperfusion injury in TKA was investigated using the IL-6 level as an index.
The study protocol adhered to the ethical guidelines of the 1975 Declaration of Helsinki, and the institutional review board of the Faculty of Medicine, Saga University at Saga, approved this study.
MATERIALS AND METHODS
Ten patients (5 males, 5 females) undergoing TKA without an air tourniquet were recruited in this investigation. Although, TKA is usually performed using an air tourniquet at our institution, all 10 patients ultimately underwent unilateral TKA without an air tourniquet (Non-tourniquet group). They all had been diagnosed with primary osteoarthritis of the knee joint, and none had any history of any type of inflammatory arthritis, systemic inflammatory or autoimmune disorder, any type of cancer, or chronic illness. We also selected 10 age- and sex-matched control patients who underwent unilateral TKA with an air tourniquet (Tourniquet group).
The details of the patients in both groups are shown in Table 1. There were no significant differences between the two groups in these characteristics. The indication for TKA was a diagnosis of osteoarthritis of the knee joint. The following inclusion criteria for TKA were strictly applied: significant osteoarthritic changes of the knee joint on X-ray, the impairment of the ADL and QOL, and difficulty in conservative treatment. All of the patients undergoing TKA were classified as the grade 1 or 2 a according to the American Society of Anesthesiologists (ASA) physical status.
| Total | Non-tourniquet group | Tourniquet group | p value | |
|---|---|---|---|---|
| Number of patients, n | 20 | 10 | 10 | |
| Sex (male, female, n) | 10, 10 | 5, 5 | 5, 5 | |
| Age, years (mean±SD, [range]) | 73.5±6.6 (60-81) | 73.7±7.0 (60-81) | 73.3±6.6 (60-80) | 0.897 |
| Diagnosis of primary osteoarthritis, n | 20 | 10 | 10 | |
| Weight, Kg (mean±SD, [range]) | 62.4±8.1 (47.9-81.1) | 60.0±5.1 (50.6-67.5) | 64.7±10.0 (47.9-81.1) | 0.207 |
| Height, cm (mean±SD [range]) | 155.5±7.1 (142.2-168.0) | 155.9±4.3 (151.2-163.6) | 155.2±9.4 (142.2-168.0) | 0.821 |
| BMI, kg/m2 (mean±SD [range]) | 25.8±2.6 (21.6-30.3) | 24.7±1.7 (22.1-26.9) | 26.8±3.1 (21.6-30.3) | 0.068 |
In all cases, the anesthesia team determined which the type of anesthesia that should be administered for TKA (spinal and/or epidural anesthesia). The anesthesia team was not involved in this study. Continuous epidural anesthesia was also used to provide pain relief on the first postoperative day.
In the Tourniquet group, the circumferential application of an elastic band to the lower extremity was used to ensure less intraoperative bleeding and to create a bloodless surgical field by exsanguinating the extremity; this was followed by the application of an air tourniquet. An automatic air tourniquet with a pressure of 250-300 mmHg (mean: 285 mmHg) was used.
A conventional medial parapatellar approach for TKA was selected in all of the cases of this series. Following implants were used in all of the TKA procedure: (Scorpio[Stryker Orthopaedics, Mahwah, NJ, USA] in 17 knees; NexGen [Zimmer, Warsaw, IN, USA] in 2 knees; Bi-Surface5 [Kyocera Medical Corporation, Osaka, Japan] in 1 knee). Eighteen patellae were not replaced, and two patellae were replaced by an implant. Bone cement was used to fix the implants in 6 cases, and 14 implants were fixed without bone cement. A 1/8-inch silicon wound drain with vacuum suction was placed in the joint capsule before wound closure. The drain was removed on the second postoperative day. Flurbiprofenaxetil, diclofenac sodium, pentazocine, and loxoprofen sodium administered as determined by the physician in charge.
Venous blood samples were obtained at 3 points (before surgery, 24 h after surgery, and 7 days after surgery) during a routine inspections, and at the time of routine clinical rounds at our institution; the serum IL-6 level was also measured at these time points. The blood samples were centrifuged for 7 min at 3500 rpm to separate the serum. The supernatant was stored at −25 °C until the sample analysis. An enzyme-linked immunosorbent assay (ELISA) was used to determine the serum IL-6 levels (BioSource Human IL-6 ELISA kit; BioSource, Camarillo, CA, USA). The ELISA was performed in accordance with the manufacturer’s instructions. The assay had a sensitivity of 2–500 pg/ml for IL-6. The total WBC count and CRP and creatine phosphokinase (CPK) level were determined in all samples. We checked the body temperature of the patients three times per day. The maximum daily temperature was investigated in this study.
The clinical findings before surgery and at one year after surgery were assessed with the Knee Society score (KSS) [19], Japanese language version which has been validated [20]. The KSS consists of the Knee Society knee score (KSKS) and the Knee Society function score (KSFS). In all patients, the possibility of surgical site infection was ruled out at one year after surgery.
Statistical Analysis
The analysis of the change in the mean levels of IL-6, CRP, CPK, and the WBC counts and maximum daily temperatures were investigated using a one-way analysis of variance (ANOVA) (before surgery vs. 24 h and 7 days after; and 24 h vs. 7 days after). The mean values of the two groups were compared using Student’s t-test. The use of cement was compared using a chi-squared test. All of the statistical analyses were performed using the SPSS for Windows software program (Version 22; IBM Corp, Armonk, NY, USA). P values of <0.05 were considered to indicate statistical significance.
RESULTS
No surgery- or anesthesia-related complications, including pneumonia, atelectasis, urinary tract infection, symptomatic thromboembolic event, or wound infection, were observed. Complete data of KSKS and KSFS were not obtained from three patients. The average KSKS for the patients indicated improvement from 34.4 (range, 10–61) before surgery to 92.0 (range, 62–100) at 1 year after surgery (p<0.01), and the average KSFS improved from 27.4 (range-20–50) before surgery to 73.8 (range, -20–100) at 1 year after surgery (p<0.01). There was no significant difference between the Non-tourniquet group and the Tourniquet group in the KSKS or KSFS values before surgery (p = 0.996 and 0.753, respectively) and at 1 year after surgery (p = 0.936 and 0.314, respectively). No surgical-site infections were observed at one year after surgery.
Table 2 shows the perioperative results and the comparative data of each group. There were no significant differences in the total blood loss or the postoperative blood loss between the Non- tourniquet and Tourniquet groups.
| Total | Non-tourniquet group | Tourniquet group | p value | |
|---|---|---|---|---|
| Operating time, minutes (mean±SD [range]) | 77.9±16.5 (51-115) | 76.7±13.0 (58-100) | 79.1±20.1 (51-115) | 0.755 |
| Intraoperative blood loss, g (mean±SD [range]) | 183.3±101.0 (50-340) | 0 | ||
| Postoperative blood loss, g (mean±SD [range]) | 869.5±231.7 (410-1210) | 873.0±218.1 (470-1180) | 866.0±256.5 (410-1210) | 0.948 |
| Total blood loss, g (mean±SD [range]) | 961.2±238.2 (410-1285) | 1056.3±184.0 (750-1285) | 866.0±256.5 (410-1210) | 0.073 |
| Blood transfused (autologous, allogenic, both, none) | 16, 2, 1, 1 | 10, 0, 0, 0 | 6, 2, 1, 1 | |
| Pressure of air tourniquet, mmHg (mean±SD [range]) | 0 | 285.0±18.4 (250.0-300.0) | ||
| Duration of air tourniquet, minutes (mean±SD [range]) | 0 | 79.6±20.1 (51.0-115.0) | ||
| Use of cement, n (with, without) | 6, 14 | 1, 9 | 5, 5 | 0.051 |
In the Non- tourniquet group, all of the cases received autologous blood transfusion. In the Tourniquet group, autologous blood transfusion was performed in 6 cases, allogenic blood transfusion in 2 cases, both allogenic and autologous blood transfusion in 1 case, and no transfusion in 1 case.
There were no significant differences in the WBC count, body temperature, or levels of CRP, CPK, or IL-6 between the two groups at any time point measured (Table 3).
| Total | Non-tourniquet group | Tourniquet group | p value | ||
|---|---|---|---|---|---|
| WBC count, /μl (mean±SD [range]) | Before surgery | 6120±1330 (2900-8600) |
6280±910 (5400-8000) | 5960±1689 (2900-8600) | 0.604 |
| 24 h after surgery |
7940±1565 (5200-10400) |
8070±1521 (5900-10400) | 7810±1678 (5200-10100) | 0.721 | |
| 7 days after surgery |
6165±1092 (4200-8100) |
6260±1141 (4600-8000) | 6070±1092 (4200-8100) | 0.708 | |
| CRP, mg/dl (mean±SD [range]) | Before surgery | 0.1±0.1 (0-0.3) |
0.1±0.1 (0.0-0.2) |
0.1±0.1 (0.0-0.3) |
0.337 |
| 24 hours after surgery |
2.4±1.4 (0.9-7.0) |
2.2±0.9 (1.0-3.6) |
2.7±1.7 (0.9-7.0) |
0.452 | |
| 7 days after surgery |
4.4±2.5 (0.9-10.5) |
3.6±2.3 (0.9-7.7) |
5.2±2.6 (1.0-10.5) |
0.163 | |
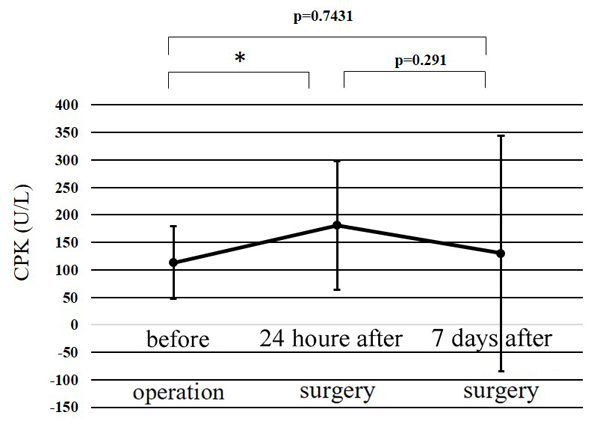
| CPK, U/L (mean±SD [range]) | Before surgery | 113±66 (38-255) |
118±68 (63-255) |
108±67 (38-239) |
0.754 |
| 24 h after surgery |
181±117 (58-508) |
147±74 (64-288) |
216±143 (58-508) |
0.192 | |
| 7 days after surgery |
130±214 (28-972) |
116±106 (33-331) |
144±291 (28-972) |
0.774 | |
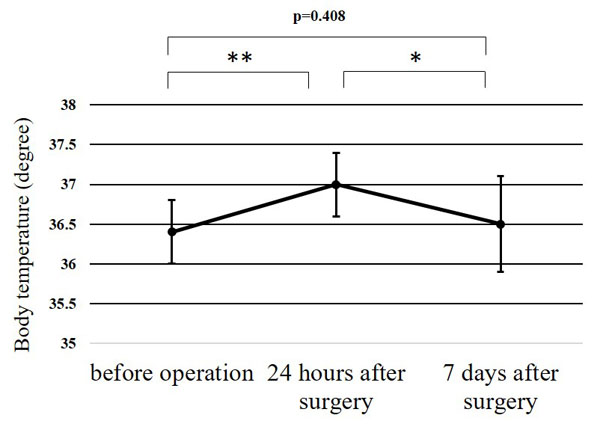
| Body temperature, °C (mean±SD [range]) | Before surgery | 36.4±0.4 (35.5-37.0) |
36.3±0.4 (35.6-36.8) |
36.4±0.4 (35.5-37.0) | 0.684 |
| 24 h after surgery |
37.0±0.4 (36.0-37.6) |
36.9±0.4 (36.0-37.6) |
36.9±0.3 (36.4-37.4) | 0.954 | |
| 7 days after surgery |
36.5±0.6 (35.2-37.6) |
36.4±0.6 (35.2-37.6) |
36.6±0.6 (35.8-37.5) | 0.696 | |
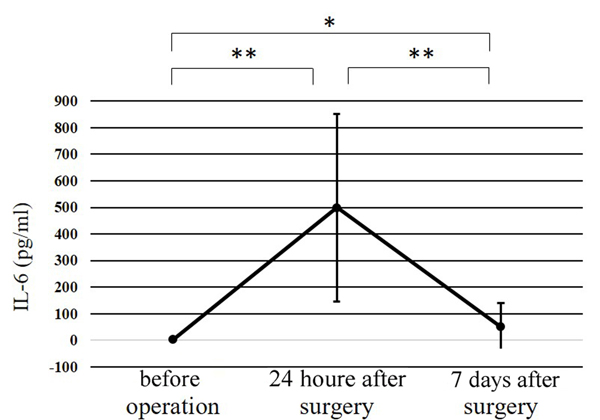
| IL-6 in serum, pg/ml (mean±SD [ange]) | Before surgery | 3.1±4.4 (0.0-17.2) |
3.9±2.8 (0.0-8.0) |
2.3±5.6 (0.0-17.2) |
0.454 |
| 24 h after surgery |
499.9±353.3 (150.2-1499.3) |
515.7±389.5 (150.2-1499.3) |
484.1±333.7 (178.9-1355.8) |
0.848 | |
| 7 days after surgery |
50.1±89.3 (3.1-400.0) |
70.8±123.3 (10.4-400.0) |
29.5±26.5 (3.1-80.7) |
0.314 | |

The WBC counts peaked at 24 h after surgery, and the peak level was significantly higher than at any other point (p<0.01) (Fig. 1). The CRP level at 24 h after surgery was significantly higher than before surgery (p<0.01); while that at 7 days after surgery was significantly greater than at 24 h after surgery (p<0.01) (Fig. 2). Significant differences were observed in the CPK levels before surgery and at 24 h after surgery (p<0.05) (Fig. 3). The body temperature peaked at 24 h after surgery, and the peak level was significantly greater than at any other point (p<0.01 vs. before surgery, p<0.05 vs. 7 days after surgery) (Fig. 4). The IL-6 peaked at 24 h after surgery, and the peak level was significantly greater than that at any other point (p<0.01) (Fig. 5).

DISCUSSION
IL-6 is mainly produced by followed by monocytes, macrophages, T lymphocytes, endothelial cells, fibroblasts and other types of cells in response to antigen stimulation [5]. The release of IL-6 from these cells is directly encouraged by surgical stress, and other locally released cytokines also promote the release of IL-6; for example, IL-1 or TNFα up-regulate the release of IL-6 from fibroblasts [21]. However, it is often difficult to detect the concentrations of TNFα, IL-1β, and IL-8 due to their low concentrations. Even in severely infected patients, it often difficult to detect IL-1β and TNFα in the systemic circulation [22]. Thus, IL-6 has come to be recognized as a major endogenous protein mediator in the acute-phase response after surgery [21]. One of the important roles of IL-6 is to induce and control the acute-phase protein response. It is well known that human hepatocytes synthesize CRP [1].
In surgery of the limbs, including TKA, an air tourniquet is used to reduce blood loss or keep the operative field clear. However, the effectiveness and safety of air tourniquets have been debated, with respect to blood loss, operation time, deep venous thrombosis, pulmonary embolism, knee swelling, duration of hospital stay, and clinical outcomes, among other factors [23]. In the current study, symptomatic deep venous thrombosis was not observed in either group, and there were no significant differences in the clinical results of the two groups. Furthermore, there were no significant differences between the two groups with regard to the perioperative factors, the operative time or the blood loss.
IL-6, which is increases at the surgical site, diffuses into systematic circulation, and the IL-6 in the systematic circulation triggers leukocytosis and a fever [24]. In joint replacement surgery, the IL-6 level peaks at to 6–24 h after surgery [10]. The timing of the peak depends on the surgical procedure [10]. It has been reported that there are significant differences in the local and systemic cytokine responses after surgery [25]. In some reports, the concentration of IL-6 at the surgical site in patients undergoing surgery for thoracic scoliosis or TKA, was up to 1000-fold greater than that in systematic circulation [11, 25]. Moreover, a complex inflammatory cascade was initiated by traumatic tissue damage, extremity ischemia and reperfusion injury to both the ischemic tissues and the tissues outside the ischemic field [26-29]. In animal experiments, IL-6 was also produced by ischemia and reperfusion [30]. In the clinical setting, the cytokine levels were found to be significantly increased at reperfusion following the application of an air tourniquet during surgery [31].
The initial symptoms of morphological injury to muscular cells were found to occur at 1 h after ischemia, and the damage of muscular cells grew worse relative to the ischemic time [32]. Ischemic injury also manifests at leakage into the extracellular and vascular spaces because the cell membrane immediately displays abnormal permeability to cytoplasmic enzymes. Furthermore, reperfusion may lead to secondary cell damage. After the ischemic period, free oxygen radicals induce many kinds of tissue damage, such as the disturbance of intracellular homeostasis, lipid peroxidation, membrane disintegration, and DNA damage, with the apoptosis and necrosis of endothelial, parenchymal, and immune cells [33, 34].
It is consistently reported that the concentration of IL-6 in systemic circulation increases after major trauma; however, the action of IL-6 has not been fully elucidated. A positive correlation is observed between the degree of tissue damage and the levels of IL-6, and the amount of tissue damage reflects the subsequent decrease of IL-6 from the first day after injury [35].
Reperfusion injury, which involves the expansion of local tissue damage, is the next step in the inflammatory response in ischemia-damaged tissue. It is know that the coagulation system is at least one of the systems responsible for the release of inflammatory mediators [36].
To our knowledge, this is the first report to compare the effect of an air tourniquet on increased postoperative IL-6 level using a control group through day 7 after surgery.
We noted that there were no significant differences between the two groups in the serum levels of IL-6, CRP, or CPK, or in the WBC count or body temperature at any of the evaluated time points. The serum level of IL-6 might increase due to extremity ischemia or reperfusion in the early phase after TKA, but the stress due to surgery itself influenced this change to a much greater degree than the air tourniquet. Therefore, when investigating the effect of air tourniquet, reperfusion injury due to air tourniquet may leave out of consideration.
Several limitations associated with the present study warrant mention. First, the study group was relatively small (10 in each group, 20 total). Second, the venous blood samples were obtained at only three points (once before and twice after surgery), and, the first sample after surgery wasn’t obtained until 24 h after surgery. We may therefore have missed evaluating the true peak level of IL-6. Third, we measured only IL-6 among many cytokines. Fourth, this was a case-controlled study and not a randomized controlled study. Further investigations that expand on our own will be needed to confirm our findings.
CONCLUSION
Our findings indicate that the effect of ischemia and reperfusion due to air tourniquet on increasing the IL-6 level was not much significant than that induced by surgical stress in TKA.
LIST OF ABBREVIATIONS
| CPK | = Creatinephosphokinase |
| CRP | = C-reactive protein |
| IL-6 | = Interleukin-6 |
| KSFS | = Knee Society function score |
| KSKS | = Knee Society knee score |
| KSS | = Knee Society score |
| TKA | = Total knee arthroplasty |
| WBC | = White blood cell |
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors thank Dr. Jun Ito for his valuable contributions to this study.


