RESEARCH ARTICLE
Tranexamic Acid in a Multimodal Blood Loss Prevention Protocol to Decrease Blood Loss in Revision Total Knee Arthroplasty: A Cohort Study#
Miguel Ortega-Andreu1, Gloria Talavera1, Norma G. Padilla-Eguiluz2, Hanna Perez-Chrzanowska3, Reyes Figueredo-Galve3, Carlos E. Rodriguez-Merchán2, Enrique Gómez-Barrena2, *
Article Information
Identifiers and Pagination:
Year: 2016Volume: 10
First Page: 439
Last Page: 447
Publisher ID: TOORTHJ-10-439
DOI: 10.2174/1874325001610010439
Article History:
Received Date: 04/05/2016Revision Received Date: 22/05/2016
Acceptance Date: 19/06/2016
Electronic publication date: 23/09/2016
Collection year: 2016

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution-Non-Commercial 4.0 International Public License (CC BY-NC 4.0) (https://creativecommons.org/licenses/by-nc/4.0/legalcode), which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Purpose:
To clarify if blood loss and transfusion requirements can be decreased in revision knee surgery through a multimodal blood loss approach with tranexamic acid (TXA)
Patients and Methods:
A retrospective study was designed in 87 knees (79 patients) that received a knee revision between 2007 and 2013. To avoid heterogeneity in the surgical technique, only revisions with one single implant system were included. A treatment series of 44 knees that received TXA and other techniques in a multimodal blood loss protocol was compared to a control series of 43 knees that received neither TXA nor the rest of the multimodal blood loss protocol. No differences in the complexity of surgeries or case severity were detected.
Results:
A significant decrease was observed from 58% transfusion rate in the control group to 5% in the treated group. The postoperative haemoglobin drop was also significantly different. Although the use of a blood loss prevention approach including TXA was the most relevant factor in the transfusion risk (OR=15), longer surgical time also associated an increased risk of transfusion (OR=1.15).
Conclusion:
This study supports the use of a two-dose intravenous TXA under a multimodal blood loss prevention approach in revision knee replacement with significant reduction in the transfusion rate, postoperative blood loss and haemoglobin drop.
INTRODUCTION
Tranexamic acid (TXA) is widely used today in primary total knee replacement (TKR). Evidence level I studies including metaanalysis and systematic reviews [1-5] have confirmed the role and efficacy of TXA to decrease transfusion rate and blood loss in primary TKR. This has lead to a minimum transfusion rate close to 0% in centres with multimodal protocols that include preoperative haemoglobin raised over 13gr/dL [6]. In all the published studies, with thousands of participating patients, no increase in distal venous thrombosis (DVT) or thromboembolic disease (TED) risks have been observed.
Revision total knee replacement (RKR) surgery has long been associated with higher blood loss and transfusion requirements than primary TKR. Although there is limited information about the use of TXA in knee revision surgery, earlier studies [7, 8] have also confirmed a decrease in blood loss. Transfusion rate dropped from 62% to 32% with 2 doses of 1 gr i.v. TXA [7], and from 30.3% to 16.7% with one intraoperative dose of 20mg/kg of TXA [8]. However, many preoperative variables such as haemoglobin, surgical variables such as tourniquet and surgical time that may express surgical complexity, or even variables such as bone defects and the reconstruction technique may widely affect these results. Such variability may justify that tranexamic acid has not been widely implemented [9], and is rarely considered in revision total knee replacement surgery.
To further clarify these issues, where very limited information is available in the literature, we set the hypothesis of the study in that the administration of TXA in a multimodal blood loss prevention protocol may substantially decrease the transfusion needs in revision total knee replacement surgery. The primary objective was to evaluate the blood transfusion rate and the blood loss in a study series of RKR that received two-dose 15mgr/kg intravenous TXA, compared with a control series of RKR performed without TXA, as per standard of care. A secondary objective was set to clarify if other preoperative variables, surgical time and reconstruction technique may affect blood transfusion and blood loss in RKR, with or without TXA.
| Variables |
Treatment group (with TXA and MBLPA) (n=44) |
Control group (without TXA or MBLPA) (n=43) |
P value | ||
|---|---|---|---|---|---|
|
Mean ± SD n(%) |
(Max-Min) |
Mean ± SD n(%) |
(Max-Min) | ||
| Age | 68.8±9.2 | (33.0-81.6) | 74.2±7.2 | (60.0-90.6) | 0.002† |
| Weight | 79.2±12.9 | (53.0-108.1) | 77.9±18.2 | (50.0-168.0) | 0.376* |
| Female sex | 32 (73%) | 32 (74%) | 0.526‡ | ||
| BMI | 30.9± 4.5 | (23.4-41.9) | 29.8± 6.7 | (18.8-61.7) | 0.097* |
| BMI category | |||||
| Normal-preobesity | 17 (41%) | 20 (51%) | 0.745‡ | ||
| Obesity II | 23 (56%) | 18 (46%) | |||
| Obesity III | 1 (2%) | 1 (3%) | |||
| ASA | 2.3±0.5 | (1.0-3.0) | 2.5±0.5 | (2.0-3.0) | 0.106† |
| ASA category | |||||
| I | 1 (2%) | 0 -- | 0.183‡ | ||
| II | 28 (67%) | 23 (53%) | |||
| III | 13 (31%) | 20 (47%) | |||
| Preoperative haemoglobin (g/dL) | 14.1±1.0 | (12.6-16.7) | 13.8±1.3 | (11.1-16.6) | 0.180† |
| Haemoglobin category | |||||
| >13 | 40 (91%) | 29 (69%) | 0.019‡ | ||
| 12-13 | 4 (9%) | 10 (24%) | |||
| <12 | -- -- | 3 (7%) | |||
| Preoperative haematocrit (%) | 43.1±2.8 | (38.3-49.5) | 42.0±3.8 | (34.7-49.3) | 0.113† |
PATIENTS AND METHODS
A retrospective cohort study was designed to analyse knee revision surgeries performed at our Hospital between 2007 and 2013. A series of 87 knees were included in the final analysis, after selecting only those cases that received Legacy Condylar Constrained Knee modular implants (Zimmer, Warsaw IN, USA) to avoid heterogeneity due to surgical and implant technique. Furthermore, we only included in the series under analysis the surgical revisions where both components were retrieved and revision components were implanted both in the femur and the tibia.
In this series, 44 knees in 38 patients were the treatment group that received tranexamic acid (Amchafibrin™, Rottapharm, Barcelona, Spain) in a multimodal blood loss prevention approach (MBLPA) developed in one of our units and previously published [6]. Informed consent for revision surgery included blood saving measures with MBLPA under use. Also during the same time frame, 43 knees in 41 patients were considered the control series as the revision surgeries were performed in a different unit following the standard of care in our Hospital at that time (without TXA and without MBLPA).
| Variables |
Treatment group (with TXA and MBLPA) (n=44) |
Control group (without TXA or MBLPA) (n=43) |
P value | ||
|---|---|---|---|---|---|
|
Mean ± SD n(%) |
(Max-Min) |
Mean ± SD n(%) |
(Max-Min) | ||
| Months survivorship of previous implant |
67.7±63.6 | (7.6-253.0) | 58.4±52.6 | (2.7-242.8) | 0.480* |
| Surgical time (min) | 114.3±22.9 | (55.0-195.0) | 136.2±39.6 | (70.0-265.0) | 0.003* |
| Tourniquet time (min) | 107.9±15.4 | (70.0-140.0) | 107.2±20.3 | (60.0-150.0) | 0.863† |
| Revision diagnosis | |||||
| Aseptic loosening | 31 (70%) | 31 (72%) | 0.734‡ | ||
| Pain | 3 (7%) | 3 (7%) | |||
| Instability | 7 (16%) | 3 (7%) | |||
| Polyethylene wear | 2 (5%) | 3 (7%) | |||
| Prosthesis malposition | 1 (2%) | 2 (5%) | |||
| Fracture | 0 -- | 1 (2%) | |||
| Femoral defect type | |||||
| 1 | 7 (17%) | 10 (23%) | 0.720‡ | ||
| 2A | 24 (57%) | 19 (44%) | |||
| 2B | 9 (21%) | 11 (26%) | |||
| 3 | 2 (5%) | 3 (7%) | |||
| Tibial defect type | |||||
| 1 | 19 (48%) | 10 (23%) | 0.064‡ | ||
| 2A | 14 (35%) | 19 (44%) | |||
| 2B | 6 (15%) | 8 (19%) | |||
| 3 | 1 (3%) | 6 (14%) | |||
| Left side | 21 (50%) | 21 (53%) | 0.498‡ | ||
Treatment in the series under investigation included two intravenous doses of TXA (15mg/Kg in 100mL of 0.9% saline solution, physiological saline solution Grifols™, Barcelona, Spain, before tourniquet release repeated three hours after surgery), as per prior publications [6, 13, 15], and a MBLPA that included the use of a pneumatic tourniquet with 100mmHg above systolic pressure, released after skin closure; preoperative Hb optimization; single 12 mm drain (Drenofast CH-12/4,0 Iberhospitex, Barcelona, Spain) without vacuum, opened 2 hours after skin closure; intraarticular infiltration before closure injecting 80cc saline with 0.3mgr adrenalin, 10mgr morphine chloride, 100mgr tobramicin, 6mgr betametasone sodium phosphate, 6mgr betametasone acetate, 200mgr ropivacaine (30cc in posterior capsule, collateral ligaments, and medial wall before cementing, 50cc in the synovium, arthrotomy and subcutaneous tissues after implantation). Patients with allergy or contraindication to TXA, major comorbidities (severe ischemic cardiopathy, sleep apnea syndrome, severe pulmonary disease, severe renal insufficiency, or hepatic failure), coagulopathy (preoperative platelet count <150,000/mm3, INR >1.4, or prolonged partial thromboplastin time >1.4 times normal), history of arterial or venous thromboembolic disease (cerebrovascular accident, deep venous thrombosis, or pulmonary thromboembolism), haematologic disorders (haematopoietic, hemorragic and thrombogenic diseases), or retinopathy (severe vision field limitation and colour distorsion), did not receive TXA. Acetylsalicylic acid, anti-platelet agents, or nonselective cyclooxygenase inhibitors were discontinued at least seven days prior to surgery. Discontinuation was confirmed upon surgical admission.
The control group included 43 knees in 41 patients that underwent knee revision surgery under standard care at the time in our Hospital. Differences with the treatment group included pneumatic tourniquet with 350mmHg that was released after implantation and cement setting, and before skin closure, for electrocoagulation of bleeding; no preoperative limits to preoperative haemoglobin and no particular treatment even in the case lower haemoglobin; 48h vacuum drain, opened immediately after skin closure; no intraarticular infiltration and no TXA administration. Therefore, these patients did not receive the multimodal blood loss prevention protocol.
The preoperative variables under consideration include patient demographics (age, gender, BMI), ASA (American Society of Anaesthesiology grading), and preoperative haemoglobin. Also, surgical and reconstruction variables analysed in both groups include side of the knee, surgical time, tourniquet time, survivorship of the previous implant that was revised during index surgery, revision diagnosis, bone defect in proximal tibia (as per the Anderson Orthopaedic Research Institute –AORI- classification [10], and bone defect in distal femur (as per AORI classification [10]. The baseline patient and case characteristics in both groups is provided in Table 1, and those of surgeries in Table 2.
Among the outcome variables, we collected haemoglobin at 24 hours, 48 hours, and 5 days; haematocrit at 24 hours, 48 hours, and 5 days; blood loss in the drain at 24 hours; blood transfusion rate and number of transfused concentrate units (where transfusion was indicated at 8 gr/dL in asymptomatic and 10 gr/dL in symptomatic or cardiac patients); length of stay in Hospital until discharge (Tables 3 and 4).
| Variables |
Treatment group (with TXA and MBLPA) (n=44) |
Control group (without TXA or MBLPA) (n=43) |
P value | ||
|---|---|---|---|---|---|
|
Mean ± SD n(%) |
(Max-Min) |
Mean ± SD n(%) |
(Max-Min) | ||
| Drain 24h (mL) | 256.4±266.6 | (20.0-1160.0) | 594.6±422.3 | (40.0-2030.0) | 0.009* |
| Transfusion (Yes) | 2 (5%) | 25 (58%) | 0.000‡ | ||
| Transfusion origin | |||||
| Allogeneic | 1 (1%) | 17 (68%) | 0.026‡ | ||
| Drain-recovery | 1 (1%) | 5 (20%) | |||
| Both | 0 -- | 3 (12%) | |||
| Number of units | |||||
| 1 | 0 -- | 8 (32%) | 0.658‡ | ||
| 2 | 2 (100%) | 13 (52%) | |||
| 3 | 0 -- | 2 (8%) | |||
| 4 | 0 -- | 2 (8%) | |||
| Lenght of stay | 6.5± 2.1 | (2.0-13.0) | 10.4± 4.3 | (3.0-22.0) | 0.000* |
The statistical methods included parametric and non-parametric statistics (Fisher test, t-test, Mann-Whitney test) that were used to compare the variables with a confidence level of 95%. A logistic regression was modelled to identify the risk factors of blood transfusion after RKR, and ORs were obtained. Furthermore, the probabilities of transfusion were then estimated after the significant logistic regression model was obtained. The statistical analysis was made using STATA software (StataCorp.2009 College Station, TX, USA).
| Variables |
Treatment group (with TXA and MBLPA) (n=44) |
Control group (without TXA or MBLPA) (n=43) |
P value | ||
|---|---|---|---|---|---|
|
Mean ± SD n(%) |
Mean ± SD n(%) |
Mean ± SD n(%) |
(Max-Min) | ||
| Postoperative laboratory tests | |||||
| Hb 24h (g/dL) | 11.8±1.1 | (9.1-13.7) | 10.7±1.1 | (8.3-13.1) | 0.000† |
| Hb 48h (g/dL) | 11.2±1.2 | (8.4-13.4) | 9.7±1.6 | (7.1-14.4) | 0.000† |
| Hb 5d (g/dL) | 11.4±2.0 | (8.5-15.2) | 10.4±1.4 | (7.8-13.4) | 0.073† |
| Haematocrit 24h (%) | 35.9±3.3 | (27.3-42.4) | 32.2±3.4 | (26.3-38.3) | 0.000† |
| Haematocrit 48h (%) | 34.1±3.7 | (26.0-41.7) | 30.0±5.1 | (20.7-45.9) | 0.000† |
| Haematocrit 5d (%) | 34.8±5.9 | (24.8-44.4) | 30.5±8.6 | (2.1-41.5) | 0.103* |
| Laboratory test differences with preoperative determinations | |||||
| Hb 24h (g/dL) | -2.4±1.2 | -(4.8-0.5) | -3.2±1.6 | -(7.3-0.0) | 0.006† |
| Hb 48h (g/dL) | -2.9±1.3 | -(5.1-0.2) | -3.9±1.7 | -(8.2- -0.7) | 0.007† |
| Hb 5d (g/dL) | -2.8±1.9 | -(5.5-1.3) | -3.4±1.8 | -(5.9-0.4) | 0.267† |
| Haematocrit 24h (%) | -7.2±3.9 | -(14.9-1.8) | -10.1±4.5 | -(21.9- -0.8) | 0.002† |
| Haematocrit 48h (%) | -9.0±3.9 | -(15.7-0.8) | -11.3±5.1 | -(27.4- -3.1) | 0.030† |
| Haematocrit 5d (%) | -8.2±5.6 | -(17.7-1.8) | -11.1±8.7 | -(38.1-2.2) | 0.256* |
RESULTS
The transfusion rate dropped significantly (p<0.001) in the study group (2 in 44, 5%), compared with the standard group (25 in 43, 58%), as seen in Table 3. Preoperative haemoglobin was not significantly different between both groups, although more cases with haemoglobin under 12 g/dL were preoperatively detected in the control group (Table 1).
Blood loss as measured in the drain at 24 hours significantly differ in both groups, confirming more visible blood loss in the control group without the multimodal blood loss prevention approach (Table 3). Significantly more haemoglobin was seen in the study group at 24 hours (11.8±1.11 vs. 10.3±1.32 mg/dL; p=0.000), and so occurred at 48 hours, while the difference between both groups was not significant (p=0.073) at 5 days, when cases without the MBLPA have already received transfusion (Table 4). Similar differences were seen in this Table 4 about haematocrit, and also when considering the postoperative drop of haemoglobin (see also Fig. 1) and haematocrit.
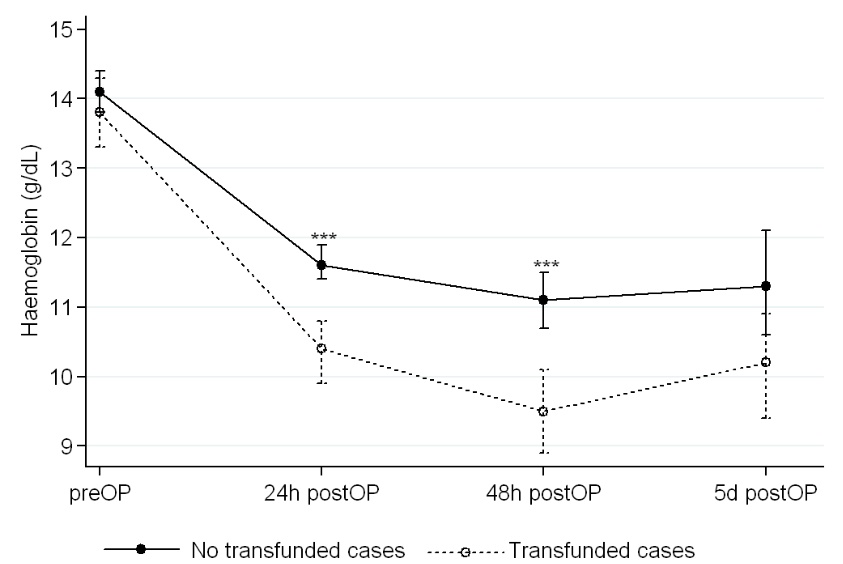 |
Fig. (1). Perioperative evolution of haemoglobin in revision knee replacements with and without transfusion.
*statistically significant differences (p<0.001).
|
A significant logistic regression model (pseudoR2=0.724) about the transfusion risk showed that the main factor to avoid transfusion was the use of TXA (OR=15), while the surgical time also influenced the risk of transfusion (OR=1.15), increasing the transfusion risk with increased surgical time (Table 5). This further allowed to derive the estimated probability of receiving transfusion in our series, depending on the surgical time (Fig. 2), and longer times of surgery up to 200 minutes clearly associates higher probability of receiving blood transfusion.
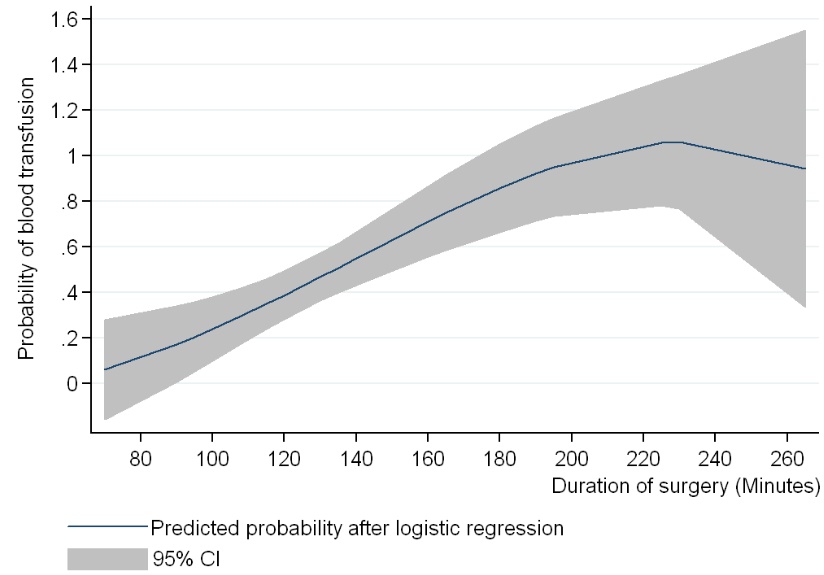 |
Fig. (2). Probability estimate. Surgical time as a predictor of transfusion after RKR in our institution (Madrid, 2007-2013). |
| Variable | OR | (CI, 95%) | P Value | |
|---|---|---|---|---|
| Age | 1.07 | (0.9- | 1.2) | 0.400 |
| Gender | ||||
| Female | 1 | |||
| Male | 0.03 | (0.1- | 1.2) | 0.060 |
| BMI | 1.15 | (0.9- | 1.5) | 0.214 |
| ASA | ||||
| I-II | 1 | |||
| III-IV | 2.74 | (0.3- | 22.3) | 0.397 |
| Preoperative Hb (g/dL) | ||||
| >13 | 1 | |||
| 12-13 | 0.05 | (0.0- | 2.4) | 0.135 |
| <12 | 0.08 | (0.0- | 22.5) | 0.381 |
| Drain (mL) | 1.00 | (1.0- | 1.0) | 0.017 |
| Surgical time (min) | 1.15 | (1.0- | 1.3) | 0.013 |
| Tourniquet time (min) | 0.87 | (0.8- | 0.9) | 0.040 |
| TXA + MBLPA | ||||
| Yes | 1 | |||
| No | 15.0 | (1.2- | 184.3) | 0.034 |
DISCUSSION
We could verify in this case-control retrospective study that significant decrease in blood loss or transfusion was achieved with TXA (2 i.v. doses) and MBLPA after RKR, standardizing the surgical technique and the implant. Furthermore, the observed transfusion rate in our study series dropped about 10 times, from 58% to 5%. The reduction in the transfusion rate is in accordance with previous publications on the use of TXA in RKR, (7, 8) but strongly expanding the blood loss control compared with the previously achieved transfusion rate decrease in the mentioned publications that was merely about one half of the transfusion rate without TXA (from 62 to 32% (7) and from 32 to 16% (8)). In part, the use of two intravenous doses could explain the higher efficacy in our results, if compared with those [8, 11] using a single dose of i.v. TXA from 10 mg/Kg [11] to 20 mg/kg (8). The support of a comprehensive blood loss prevention protocol is also an important part of the transfusion reduction, as has been seen in 12 knee revision cases in a recent report [12].
Limitations of our study include the fact that this is a retrospective study, while a randomized clinical trial would better confirm this difference. However, available data about blood loss prevention protocols and particularly about TXA make more difficult to perform studies against placebo in Hospitals with approved blood loss prevention protocols. Not providing TXA and blood loss prevention techniques in those Hospitals could be considered unethical. Other limitations include slight differences in age and preoperative haemoglobin, with more cases under 12 mg/dL in the control group even if the preoperative haemoglobin is not significantly different in bout groups. A preoperative raise of haemoglobin over 13gr/dL could be as beneficial as in the primary knee [13], although the number of cases in our series with preoperative low haemoglobin in the control group is low. Revision surgeries with those values could be at preoperative indication of erythropoietin [14] or intravenous iron or both.
In our results, it is coherently seen that both the drain loss at 24 hours and the decrease in haemoglobin and haematocrit at 24 and 48 hours were significantly less pronounced in the treated group (half of the blood volume in the drain and 1 g/dL less haemoglobin drop). Instead, the decrease at 5 days was compensated by the performed transfusions in the control group and the difference between groups was not significant at this point.
Direct and indirect consequences of this decrease in blood loss also deserve attention. As for the primary TKR and without incorporating specific fast-track protocols in this series, a significant decrease in the hospital length of stay is seen, translating into direct savings in addition to allogeneic transfused units (300€ per unit in our setting) [15], or blood reinfusion systems. Besides, indirect cost-savings also may be claimed [16], by decreasing transfusion associated risks (not only transmitted diseases, but also knee infection [17] or other complications).
Interestingly, and although the series was restricted to a single revision surgical technique to homogenize the potential confounders (if different intramedullary preparation or metaphyseal and soft tissue release was required), significant differences in surgical time were detected. Longer surgical time was associated with more transfusions. Although the influence of surgical time in the transfusion rate was less marked that the case of using TXA and developing the whole blood loss prevention protocol, it stood in the multiple regression model. This could be explained because in the control series with longer surgical time, tourniquet was released before skin closure for electrocoagulation. Instead, this was overall associated with more blood loss and transfusion needs. Furthermore, the surgical complexity was equivalent, and no differences were found in the case mix of different bone defects in femur and tibia, as both the treatment and the control groups included severe cases with serious bone defects, the difference being not significant. Long surgical time has also been related to infection in primary TKR [18], and also to higher revision rates if surgical time exceeded 120 minutes [19].
CONCLUSION
As a conclusion, this study confirmed that a two-dose intravenous administration of TXA under a MBLPA in revision knee replacement was effective in decreasing blood loss in the drain and the haemoglobin, and also the transfusion rate (to one tenth), confirming also the influence of a longer surgical time in associating blood transfusion. Therefore, this case-control study supports the use of a 2-dose intravenous TXA under a multimodal blood loss prevention approach in revision knee replacement.
LIST OF ABREVIATIONS
| AORI | = Anderson Orthopedic Research Institute |
| ASA | = American Society of Anaesthesiology |
| BMI | = Body mass index |
| MBLPA | = Multimodal blood loss prevention |
| OR | = Odds-ratio |
| RKR | = Revision knee replacement |
| TED | = Thromboembolic disease |
| TKR | = Total knee replacement |
| TXA | = Tranexamic acid |
COMPLIANCE WITH ETHICAL STANDARDS
All procedures involving human participants were in accordance with the ethical standards at the 1964 Helsinki declaration and its later amendments for retrospective studies. All the patients gave informed consent for surgery and those in the study group were informed of blood saving techniques in use.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the support from all the medical and paramedical personnel serving the Cantoblanco Unit and the surgical block and hospitalization units of Orthopaedic Surgery and Traumatology in La Paz Hospital.








