All published articles of this journal are available on ScienceDirect.
Refixation of Osteochondral Fractures by an Ultrasound-Activated Pin System – An Ovine In Vivo Examination Using CT and Scanning Electron Microscope
Abstract
Background:
Osteochondral injuries, if not treated appropriately, often lead to severe osteoarthritis of the affected joint. Without refixation of the osteochondral fragment, human cartilage only repairs these defects imperfectly. All existing refixation systems for chondral defects have disadvantages, for instance bad MRI quality in the postoperative follow-up or low anchoring forces. To address the problem of reduced stability in resorbable implants, ultrasound-activated pins were developed. By ultrasound-activated melting of the tip of these implants a higher anchoring is assumed. Aim of the study was to investigate, if ultrasound-activated pins can provide a secure refixation of osteochondral fractures comparing to conventional screw and conventional, resorbable pin osteosynthesis. CT scans and scanning electron microscopy should proovegood refixation results with no further tissue damage by the melting of the ultrasound-activated pins in comparison to conventional osteosynthesis.
Methods:
Femoral osteochondral fragments in sheep were refixated with ultrasound-activated pins (SonicPin™), Ethipins® and screws (Asnis™). The quality of the refixated fragments was examined after three month of full weight bearing by CT scans and scanning electron microscopy of the cartilage surface.
Results:
The CT examination found almost no statistically significant difference in the quality of refixation between the three different implants used. Concerning the CT morphology, ultrasound-activated pins demonstrated at least the same quality in refixation of osteochondral fragments as conventional resorbable pins or screws. The scanning electron microscopy showed no major surface damage by the three implants, especially any postulated cartilage damage induced by the heat of the ultrasound-activated pin. The screws protruded above the cartilage surface, which may affect the opposingtibial surface.
Conclusion:
Using CT scans and scanning electron microscopy, the SonicPin™, the Ethipin® and screws were at least equivalent in refixation quality of osteochondral fragments.
BACKGROUND
Osteochondral injuries, if not treated adequately, often lead to severe osteoarthritis of the affected joint, because the cartilage itself only has poor regeneration capability [1]. Surface alignment of the opposing parts of the joint is important to prevent early osteoarthritis [2]. Osteochondral fractures, often called “flakes”, occur as completely or partiallyattached fragments. Another kind of injury is an impaction of the cartilage with a fracture of the subchondral membrane [3]. Without refixation of the osteochondral fragment, human cartilage can only repair these defects imperfectly. During healing of chondral defects, fibrous tissue is formed, which has inferior characteristics compared to intact cartilage concerning weight bearing and surface structure [4]. To prevent this imperfect repair and to achieve cartilage quality, the refixation of these osteochondral fragments should be sought.
All existing refixation systems for chondral defects have disadvantages, such that a gold-standard can not be defined [5]. Non-resorbable implants includeK-Wire, Smillie pins and cortical nails [5, 6]. These implants require a subsequent operation for removal of the implant and they impair the quality of CT and MRI imaging. Other potential problems include loosening of the implant, and the unresolved problem of elevated metal ion levels in the peripheral blood [7, 8]. Metal screws can ensure better fixation of the fragment, but may also cause cartilage damage during removal. Naturally, the stability of such screws is considerable and produces good healing results, but damage to the joint surface has been observed [9, 10].
For refixation of osteochondral fragments, histoacryl and fibrin adhesives can also be used [11]. The histoacryl gluten exhibits strong primary fixation, but long-term anchoring often fails because histoacryl has a long resorption time, causing a barrier effect. Fibrin gluten can be absorbed by the human body more quickly, but has a lower primary anchoring force [12].
With regard to resorbable implants, more than 40 resorbable polymers fororthopaedicpurposes are known. Polyglycolide (PGA), poly-L-lactide (PLLA), poly-D,L-lactide (PDLLA) and polydioxanone (PDA) are often used. By hydrolytic metabolism, these implants can be broken down into water and CO2 in the citric acid cycle [7, 13, 14]. The biocompatibillity of these implants concerning the use in bone could be prooved in several studies [15, 16]. These implants often lack strong primary anchoring and long-termfixation [17]. For instance, stability against shear forces in PLLA pins dropped by 25–50% after 4 weeks [18].
The ideal resorbable implant for the refixation of osteochondral fragments should have the mechanical characteristics of metal implants regarding compression force and stability against shear forces. Most do not exhibit these characteristics [17]. To address the problem of reduced stability in resorbable implants, ultrasound-activated pins were developed. Initial tests in cranio-facial surgery showed promising results (SonicWeld®system with Resorb-X® pins) [18]. These resorbable implants are usually inserted via a drill hole. Afterwards the tip of the implant is melted into the bone by application of ultrasound oscillation.
The aim of this study was the radiological assessment of refixated osteochondral fragments by CT and scanning electron microscopy. The good biocompatibility concerning a possible heat damage of cartilage surfaces of the SonicPin™ system could be stated by another study [19]. To evaluate whether an ultrasound-activated pin (SonicPin™, Stryker, Schönkirchen, Germany) could safely refixate an osteochondral fragment under physiological conditions, 16 merino sheep were operated. To compare the SonicPin™ to a resorbable non-ultrasound-activated pin, we used the Ethipin® System (ETHICON, Norderstedt, Germany). For comparison to conventional screw fixation, we used the cannulated titanium Asnis™ screw (Stryker, Schönkirchen, Germany). Sheep, as an animal model, have been used successfully in several studies concerning the examination of cartilage [20-22]. The anatomy and the low flexion position of the sheep knee are similar to humans. Furthermore, the biomechanical forces are analogous to those in the human knee [23].
METHODS
The SonicPin™ consists of a copolymer of Poly-(L-Lactid-co-D and L-Lactid) in a proportion of 70:30. The SonicPin™ has a diameter of 1.8 - 2.2 mm and is available in lengths of 18 – 22mm (2.2 mm diameter and 18mm length used in this study). For application, the pin is fixed with its proximal thread on the ultrasound applicator. Subsequently the pin can melt into a pre-drilled hole by application of a defined amount of ultrasound energy. The SonicPin® melts with its tip deep in thebone and fillsthe lacunes of the cancellous bone. Immediately after the ultrasound application, the material cools and hardens, therefore providing secure anchoring. Afterwards, the ultrasound-applicator can easily be removed from the thread of the pin (Fig. 1).
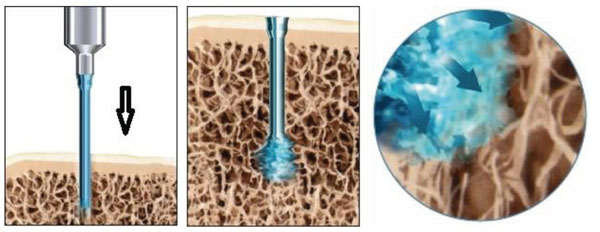
SonicPin™ mounted on the ultrasound applicator and melted in the cancellous bone after activation by ultrasound.
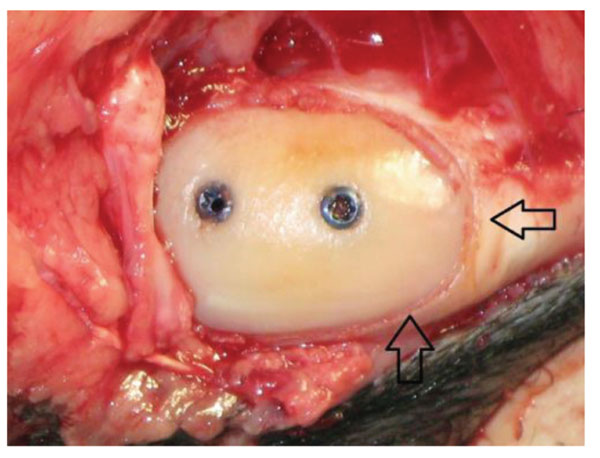
Intraoperative view on a refixed fragment (screws). The arrows mark the osteotomie gap.
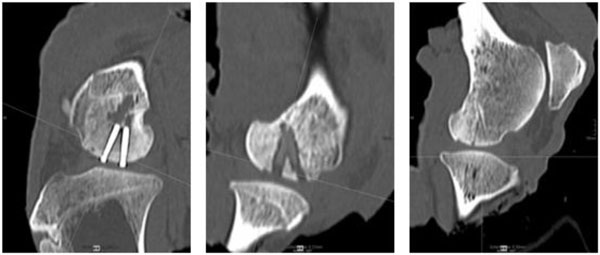
Exemplarily slices of the multiplanar CT reconstruction of the sheep knee implanted with screws (left), SonicPins™ (middle) and Ethipins® (right) showing three osteointegrated fragments of the distal femur condyle with the correspondent implant.

Scanning electron microscopy view of an osteosythesis screw from two different knees showing intact cartilage surfaces around the implant. The emerging screw head demonstrates the shrinkage of the refixed fragment.

Scanning electron microscopy view of the Ethipin®osteosynthesis screw showing intact cartilage surfaces around the implant. The scar-like parallel damages evolve from the surgical cutting of the implant during implantation are still visible after three month.
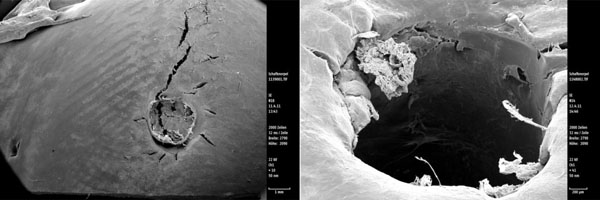
Scanning electron microscopy view of the SonicPin™ osteosythesis screw showing intact cartilage surfaces around the implants (the crack in the left picture arised during preparation). The right picture shows the look on the cartilage directly next to the removed implant.
The Ethipin® consists of Polydioxanone and has a length of 40 mm and a thickness of 1.3 mm. In comparison to the SonicPin™, the Ethipin® had no cone or change of diameter. The ostoechondral fragment is fixed by the included K-wire. Afterwards the K-wire was removed and the Ethipin®was introduced into the hole using the included application shell. If the rest of the Ethipin®was raisedabove the surface of the cartilage into the joint, it was cut away with a surgical knife at the level of the cartilage. This procedure can lead to superficial cartilage damage, if not performed carefully and constitutes a difference to the competing implants (see Fig. 5). The Ethipin® is a provenimplant for refixation of osteochondral fragments. The handling of the Ethipin® is simpleand the resorption is good, but the stability, especially for traction forces, is only moderate [24-26].
The cannulated screws (Asnis™, Stryker, Schönkirchen, Germany) that were used had a length of 16 mm and a diameter of 2 mm. The screws had a self-cutting thread and consisted of titanium. Similar to the Ethipin®, this screw was applied over the K-wire that was positioned beforehand. The lowering of the whole screw under the level of the cartilage should prevent damage to the opposing joint surface.
The study protocol was approved by the ethical committee of the Ministry for Nature and Environment of Schleswig-Holstein, Germany. In 16 Merino sheep, defined osteochondral fragments on the medial condyle of the femur were produced. The 16 sheep had a mean age of 1.5 years and a mean weight of 55.4 kg. Each hind leg of the sheep underwent surgery. The before performed biomechanical testing in laboratory and a power analysis led to a distribution of 16 SonicPin™, 8 Ethipin® and 8 screw applications. The choosen testing szenario should show the perfomance of th new SonicPin® technique versus the established refixation methods. In each sheep was at least one SonicPin® application performed, while the contralateral knee was treated with each of the conventional applications.
The operation was performed under combined anaesthesia with Rompun® by intramuscular injection and a spinal anaesthesia with Carbostesin®. Both hind legs of the sheep were operated on.
By a medial arthrotomy, the medial condyle was exposed and a defined osteochondral fragment (1 cm diameter and 4 mm thick) was produced by using a dedicated saw guide. This fragment was thenrefixated by the previously randomised implant type. Two implants were used on each osteochondral fragment. All implants used were implanted with a drill- guide which provided a 15° degree angle of the two implants to each other (Fig. 2).
A softcast and a sterile bandage were applied after finishing the arthrotomy. After 5 days, the soft cast was removed. Full weight bearing for the whole time of the study was desired as a worst-case scenario. Analgesia was done by metamizol-enriched water for at least 14 days and then till no signs of protection of the extremities could be observed. Three months after surgery, the sheep were euthanised by an overdose of barbiturates, and the knees were dissectedfor examination.
The computed tomography (MDCT) was performed using a 64-row Siemens Somatom Definition AS Scanner (Siemens Healthcare, Forchheim, Germany), with a gantry rotation time of 0.5 s, a collimation of 64 x 0.6 mm, slice thickness of 1 mm and slice increment of 0.8 mm. Image reconstruction was performed using a sharp, high-resolution kernel. The specific parameters for the MDCT were a tube voltage of 120kV, an effective tube current of 100 mAs and a pitch of 0.35. An automatic tube current modulation (CARE Dose4D, Siemens Healthcare) was not used. Subsequent image post-processing was performed on an IMPAX EE R20 VII dedicated workstation (Agfa HealthCare GmbH, Bonn, Germany). The effective radiation dose was calculated with CT-Expo V2.0© (Medizinische Hochschule Hannover). Image analysis was carried out by three independend experts; two experienced surgeons and one experienced radiologist. Image quality (IQ) was calculated on source images using line profile plots perpendicular to the longitudinal bone axis. For the evaluation of the overall diagnostic image quality, a subjective 5-point-scale was used by the readers as follows: 1= severest artifacts, bone structure not definable; 2 = severe artifacts, considerable blurring of the bone structure, cancellous structure can be noted; 3 = moderate artifacts, bone structure blurred, cancellous bone structure definable; 4 = mild blurring of bone structure, good separability of the cancellous bone structure; 5 = no artifacts, sharp image of bone and cancellous bone structure.
The following features were evaluated: the fragment position, the margin of lysis around the implant, the effect on the opposing tibial surface, the bone density and the osteotomy gap. All these features were examined on axial source images and multiplanar reconstructions. The osteotomy gap and the margin of lysis were measured in millimeters. For the fragment position and tibial surface, a subjective 5-point score was used: 5 points were documented for the best results (fragment in original position and intact) and 1 point for the worst results (fragment destroyed, bare osteotomy site). For the tibial surface, 1 point meant that the cortical surface of the tibial plateau was completely perforated (identified by radiological proof of perforation of the subchondral membrane) whereas 5 points meant that no radiological affection on the tibial surface was observed. The points in between were documented for the erosion of the cortical bone of the tibial plateau in quarters. The bone density was measured in Hounsfield-units (HU) with a comparison of the fragment that was refixated(medial condyle) and the apposing area on the lateral condyle in each knee.
After the CT scans, the cartilage surfaces were prepared for the scanning electron microscope. The preparations were fixed in a Monti-Graziadei solution (2% glutaraldehyde, 0.6% paraformaldehyde in 0.1 M cacodylatebuffer, at pH 7.2) for 2 days. The dehydration was performed by alcohol solutions of rising concentration (30, 40, 50, 60, 70, 80, 90 and 100% eachfor 15 min). After dehydratation, the samples were placed on an alloy tray and were treated with platinum. The examination itself was done with a Philips SEM 505 scanningelectronmicroscope (Philips, Eindhoven, Holland). The cartilage surface next to the implants (femur) was examined in by a qualitative description.
Statistical Analysis
The statistical analyses were performed using the Wilcoxon signed-rank test. SPSS software v20 (IBM, Ehningen, Germany) was used, and the level of significance was assumed to be p < 0.05.
RESULTS
Animals
Two sheep died before the minimum survival time of 3 months due to pneumonia. In the end 14 knees could by examined with the SonicPin™ System, 8 knees with the Asnis™ scew system and 6 knees with the Ethipin® System.
CT-Scans
The quality of the scans was not compromised. All scans could be rated regularly and wererated to 4 or 5 points and were included into the analysis. The inter-rater reliability during examination of the CT-scans was high (Fleiss´K: 0.83).
The Ethipin® group showed the smallest margin around the implant in both views (Table 1). Statistical analysis of the margin of lysis showed a significant difference between the Asnis™-screws vs the Ethipin® group and the SonicPin™ group vs the Ethipin® group for the axial as well as the multiplanar view (p-values in Table 2). The comparison of the margins of lysis of the SonicPins™ vs the screws was not significant. For reason of clarity and comprehensibility only the p-values of the statistical significant differences were stated in Table 2.
Arithmetic mean margin of lysis and the osteotomy gap of different refixation systems by CT scan including standard deviation.
| Implant | Margin of Lysis Axial (mm) | Margin of Lysis Multiplanar (mm) | Osteotomy Gap Axial (mm) | Osteotomy Gap Multiplanar (mm) |
|---|---|---|---|---|
| SonicPin™ | 1.1 ± 1.1 | 0.8 ± 0.49 | 0.5 ± 0.6 | 0.2 ± 0.5 |
| Ethipin® | 0.2 ± 0.27 | 0.2 ± 0.3 | 1.1 ± 1.4 | 0.8 ± 2.0 |
| Screws | 1.5 ± 1.19 | 1.7 ±1.19 | 0.1 ± 0.4 | 0.0 ± 0.0 |
Arithmetic mean p-Values of statistical comparison of the margin of lysis by CT-scans including standard deviation.
| SonicPin™ | Ethipin® | Asnis™-Screws | |
|---|---|---|---|
| SonicPin™ | -- | p=0.0064 (axial) p = 0.0058 (multiplanar) |
p=0.12 (axial) p = 0.15 (multiplanar) |
| Ethipin® | p = 0.0064 (axial) p = 0.0058 (multiplanar) |
-- | p = 0.0075 (axial) p = 0.0023 (multiplanar) |
| Asnis™-Screws | p = 0.12 (axial) p = 0.15 (multiplanar) |
p = 0.0075 (axial) p = 0.0023 (multiplanar) |
-- |
Arithmetic mean fragment position and tibial surface rated in points of different refixation systems by CT scan including standard deviation.
| Implant | Fragment Position Axial (Points) |
Fragment Position Multiplanar (Points) |
Tibial Surface Axial (Points) |
Tibial Surface Multiplanar (Points) |
|---|---|---|---|---|
| SonicPin™ | 3.21 ± 1.8 | 3.21 ± 1.7 | 4.21 ± 1.3 | 3.93 ± 1.5 |
| Etipin® | 3.00 ± 2.2 | 2.67 ± 1.9 | 4.33 ± 1.6 | 4.17 ± 1.6 |
| Screws | 4.13 ± 1.4 | 3.75 ± 1.3 | 3.5 ± 1.7 | 3.25 ± 1.6 |
Arithmetic mean bone densitiy measured in Hounsfield-units.
| Overall Osteotomy |
Overall Contralateral |
SonicPin™ | SonicPin™ Contralateral |
Etipin® | Etipin® Contralateral |
Screw | Screw Contralateral |
|
|---|---|---|---|---|---|---|---|---|
| HU | 820 | 636 | 829 | 625 | 653 | 547 | 931 | 674 |
| + 28% | + 32% | + 19% | + 38% |
The rating for the osteotomy gaps showed the highest results in the screw groups (Table 1), so the osteotomy gaps in the screw group were smallest due to high compression force of the screws. But the statistical comparison of the results with the other osteosynthesis methods was not significantly different for the three implants in either view.
The results for the rating of the opposing tibial surface showed better results in the resorbable implant groups than the screw group (Table 3). Understandably the non-resorable implants can harm the opposing tibial surface more than the resorbable implants because they are not a subject of degradation. But the statistical analysis showed no significant differences in both views. The results for the rating of the fragment position showed better results in the screw group than both resorbable implants (Table 3), but the statistical analysis showed again no significant differences in both views.
The bone density (HU) of the refixated fragment in comparison to the untreated lateralcondyle showed an overall rising of Hounsfield-units in the refixated fragments. The biggest rise could be stated for the Asnis™-screw group followed by the SonicPin™ and the Ethipin® group (Table 4). The measured increase of bone density showed no significant difference between the implants.
In summary no statistical significant differences between the three groups could be found concerning the comparison of the bone densitiy, the osteotomy gap, the fragment position and the tibial surface. Only the margin of lysis around the Ethipin® showed significant differences in comparison to the other implants.
Exemplary slices of the multiplanar CT reconstructions of the different implant types can be seen in Fig. (3).
Scanning Electron Microscopy
Electron microscopy images are limited to a qualitative description of cartilage. A statistical quantitative analysis is not practicable. Concerning the osteosynthesis screw, a smooth cartilage surface was found. In 2 of the 8 cases, the screws protruded above the level of the cartilage, this can be seen in Fig. (4). None of the screw heads were securely covered by cartilage tissue (Fig. 4).
In the Ethipin® group, in 2 cases the implant rose over the surface level of the cartilage. In the other 4 cases, the implant was nearly covered by cartilage tissue. Furthermore, could be observed that the cutting of the implant by the surgical knife left some marks on the cartilage tissue. A margin of lysis was not observed (Fig. 5).
Observing the SonicPin™ group, no implants protruded above the level of the cartilage surface, but none were covered by cartilage. The cartilage around the implants showed no defects. No margin of lysis was observed. After removing the implant, we saw overlapping flaps of cartilage surrounding the hole, as we did shortly after drilling (Fig. 6).
DISCUSSION
By CT and scanning electron microscopy, refixated osteochondral fragments in sheep were examined to evaluate if an ultrasound-activated pin showed different radiological outcome than conventional implants such as Ethipin® and cortical titanium screws. By CT we compared the following features: the fragment position, the margin of lysis around the implant, the effect on the corresponding tibial surface, the bone density and the osteotomy gap.
The parameters observed in the CT scans were not significantly different from each other, except for the margin of lysis measured around the Ethipin®, which was significantly smaller.
The explanation for this result lies in the way the Ethipin® was applied: it has to be applied after drilling with a K-Wire and not with a drill, with real excavation and a smaller diameter hole (1.3 mm compared to 2.0 mm drill diameter). The both other implants (screws, SonicPins®) need a real driller with excavation of debris that leads to bigger margins of lysis around the implants. Therefore, a larger margin of lysis around the screws and the SonicPin™ does not necessarily mean a stronger reaction in the surrounding tissue. Furthermore the results differ only around one millimeter and underlie a small possible error of measurement. Concerning the CT examination of the SonicPin™, this system is qualified for the secure refixation of osteochondralfragments, because the quality of refixation (CT) was similar to the srew fixation and Ethipin® osteosynthesis. Despite the fact that the radiological assessment of the tibial surface showed no significant differences, the impression of the prominent screw heads leads to the conclusion that a cartilage damage of the corresponding tibial surface has to be assumed. The screws were securely positioned under the cartilage surface during operation, so a migration of the screws or shrinking of the refixed fragment can be discussed. We could not state significant larger margins of lysis around the screws that would prove a migration of themselves, so the shrinkage of the fragment is the most probable explanation for the protrusion of the screw heads. This in vivo shrinkage finds its analogy in the risen Hounsfield-Units of the refixated fragments. When the volume of the fragments decreases the concentration of the tissue rises. An explanation for the documented shrinkage might be the postoperative full weight bearing we allowed. That leads to a high compression of the refixated fragment, but as well to certain shear forces during flexion. As above mentioned the study design was chosen as a worst case scenario by full weight bearing to test the new SonicPin® technique versus the conventional application under high load. In case of patient treatment only partial weight bearing would be allowed.
Schulman et al. showed that the ability to discriminate between bone and implant is greater with an XtremeCT, which allows a better examination of the bone-implant interface. However, an XtremeCT was not available for this study and might produce more exact results, especially when examining the tissue next to the implants [27]. Due to the higher compression force, the screws showed the best results for the osteotomy gap size, but this difference was not statistically significant. The bone density of the refixated fragments showed a higher overall density compared to the opposing lateral femur condyle, but no significant difference was seen between the implants. This observed subchondral sclerosis might depend on the fact that three months after the operation the healing process is still ongoing [28, 29]. Furthermore the compression due the postoperative full weight bearing can lead to a temporarily rising of the local calcium concentration [30].
Morgan et al. studied the possibility of predicting the possible weight bearing ability of healing fractures by CT scans of the callus [31]. In the case of osteochondral fractures, callus normally does not form, but perhaps a new study using the XtremeCT could lead to similar results for osteochondral fractures [32].
Examination of the screw group by scanning electron microscopy showed that the heads of all the screws were visible on the surface, and no screw was completely covered by cartilage. Despite the fact that all screws were initially positioned deeper than the chondral surface, 2 screws were clearly raised above the cartilage surface. Damage to the opposing tibial surface is unavoidable in these cases especially under full weight bearing. In the Ethipin® group, we observed only 2 implants raised slightly above the cartilage surface, but after three months the marks of the surgical knife were still visible. The SonicPins® were not raised above the surface of the cartilage. The different coverage of the implanted application may be explained by the larger drill holes that has to be performed in the SonicPin® and the screw application. The larger holes naturally need a longer time to be covered by cartilage. Furthermore the above discussed shrinkage of the fragment can especially in the screw applications lead to relative emerging above the cartilage surface. The implanted screw cannot migrate into the deep because of the thread and underlies not a biological degradation.
All examined implants showed no damage to the cartilage around the implants, which is often suggested to occur due to the heat arising from the drilling or the melting process for the SonicPin™ [33, 34]. Further the authors could prove in another histological study no cartilage damage by the induced heat during application of the SonicPins® (quelle19).
To the best of our knowledge, no publications to date have dealt with the assessment of ultrasound-activated pins for Refixation of osteochondral fragments via CT and scanning electron microscopy.
CONCLUSION
The features observed from the CT scans showed almost no significant differences between the treatment options. Concerning morphology observed by CT scan, ultrasound-activated pins showed at least the same quality in refixation of osteochondral fragments as conventional resorbable pins or screws. A qualitative description of the surface of osteochondral fragments by scanning electron microscopy showed protrusion of the screw heads above the surface of the cartilage. No major cartilage damage was observed for any of the three implants. A histological examination of the cartilage was not part of this study. By CT scans and scanning electron microscopy, the SonicPin™, the Ethipin® and screws are at least equivalent in refixation quality of osteochondral fragments.


