RESEARCH ARTICLE
Two-Stage Revision Total Knee Arthroplasty in Cases of Periprosthetic Joint Infection: An Analysis of 50 Cases
Leif Claassen*, Christian Plaass, Kiriakos Daniilidis, Tilman Calliess , Gabriela von Lewinski
Article Information
Identifiers and Pagination:
Year: 2015Volume: 9
First Page: 49
Last Page: 56
Publisher ID: TOORTHJ-9-49
DOI: 10.2174/1874325001509010049
Article History:
Received Date: 7/11/2014Revision Received Date: 27/1/2015
Acceptance Date: 3/2/2015
Electronic publication date: 27/2/2015
Collection year: 2015

open-access license: This is an open access article licensed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted, non-commercial use, distribution and reproduction in any medium, provided the work is properly cited.
Abstract
Objectives: A periprosthetic joint infection (PJI) is a significant complication after total knee arthroplasty (TKA). Still there is no agreement on a perfect diagnosis and treatment algorithm. The aim of this study was to evaluate the success and revision rates after two-stage revision total knee arthroplasty (TKA) and factors that affect the success rate.
Material and Methods: 50 consecutive two-stage revision TKAs were performed between January 2011 and December 2012. We retrospectively reviewed study patient's charts including demographics, prior surgeries, comorbidities, incidence of persistent infection and revisions. At the final follow-up examination the patient's satisfaction, pain level and disorders were evaluated. A successful clinical outcome was defined as a functioning prosthesis without wound healing disorders, no sinuses tracts or other clinical evidence of a persistent infection.
Results : Re-implantation of prosthesis was performed in 47 cases; three patients received a septic arthrodesis. Twelve patients had a persistent infection despite two-stage re-implantation resulting in a success rate of 76.0%. In eight of these twelve patients an infecting germ was isolated during second-stage procedure. Three patients received another two-stage revision arthroplasty and one patient an above knee amputation. A revision was performed in 23 of 50 patients (46.0%). Factors that diminish the success rate were further operations after primary TKA (p = 0.048), prior revision arthroplasties after TKA (p = 0.045), nicotine abuse (p = 0.048), Charlson comorbidity index above a score of 2 (p = 0.031) and a mixed flora during first-stage procedure (p < 0.001). Age, sex, immune status, chronic anticoagulant use, rheumatoid arthritis, body mass index and the presence of multidrug resistant germs showed no significant effect on success rate (p > 0.05).
Conclusion : We found that patients who required surgery after the primary TKA, had a higher Charlson comorbidity index or were found to have mixed flora during explantation. The treatment of PJI remains difficult, both for the patient and for the treating surgeons.
INTRODUCTION
The number of primary total knee arthroplasties (TKA) has continued to increase. Simultaneously the incidence of periprosthetic joint infection (PJI) increased. The reported rate of PJI has varied from 1-4% in large published series [1-4]. Tsukayama et al. published a now commonly used classification system for PJI [5]. Still the diagnosis of a PJI remains difficult, particularly in cases of late and chronic infection. The patient’s history and clinical examination are valuable tools for diagnosing a PJI [1, 6, 7]. Key factors of the patients’ history are: wound healing complications, wound drainage, post-operative antibiotic use for suspected infection any history of infection, prior attempts at revision and detected microorganism during revision or diagnostic joint aspiration previously. Key features of the clinical examination include: redness, swelling, warmth and loss of function in combination new onset or change in patient’s pain or elevated body temperature above 37.5°C [1, 6, 7]. Certain laboratory tests can aid obtaining a diagnosisincluding: diagnostic joint aspiration, open biopsy, serum inflammatory markers, szintigraphy, intraoperative culture and histology [1, 3, 4, 8, 9]. The accuracy of serum inflammatory markers and diagnostic aspiration is controversial [1, 10, 11]. But there is agreement on the diagnosis of a PJI if the patient has a sinus tract, positive intraoperative culture, >5 polymorpho-nuclear cells per high-powered field on histology or the presence of gross purulence during the operation [1, 2, 8, 10]. There are several different treatment algorithms described for treating a PJI [5, 7, 12-15]. Tsukayama et al. recommended a revision and antibiotic therapy for category II and III PJIs. For category I and IV PJIs a two-stage revision is considered the gold-standard for treatment. The success rates ranges from 37.1% to 100% [14]. Other have found previous infection and revision of the affected knee and comorbidities like obesity, nicotine abuse, diabetes mellitus, compromised immune status and coagulopathy to be risk factors for failure of revision TKA [5,16]. Additionally the Charlson comorbidity index seems to be associated with an increased rate of persisting infection after two-stage revision arthroplasty [6, 16].
The aim of this study was to evaluate the success rate, revision rate and factors that affect the success rate after two-stage revision TKA.
MATERIAL AND METHODS
Patients and Diagnostic Parameters
The local ethical committee approved the study. 50 consecutive cases of two-stage revision knee arthroplasties in our tertiary referral center from January 2011 to December 2012 were included in this study. Inclusion criterion was a performed two-stage revision arthroplasty within the named period. Exclusion criteria were trauma affecting the operated knee after first- or second-stage procedure or deviation from treatment algorithm. 15 knees were originally implanted in our institution. 35 were referred for revision arthroplasty.
The assessment of PJI was based on combination of patients’ history, clinical examination, serum inflammatory markers, radiographs, szintigraphy and diagnostic joint aspiration. An infection was diagnosed following the recommendations from Parvizi et al. [2]. Additionally, there were 14 cases that did not meet the conditions described by Parvizi et al. but the treating surgeon classified the combination of patients’ history, diagnostic findings and intraoperative appearance as a potential low-grade infection and a two-stage revision was performed (Table 1).
Indication parameters to perform a two-stage revision arthroplasty. The indication parameters are described in detail. It should be separated between mandatory signs of an infection like elevated body temperature in combination with local signs of an infection, draining sinus, purulence and detected microorganisms in diagnostic joint aspiration or previously performed revisions. And relative signs of infection that have to be correlated with other signs like patient history, elevated CRP, isolated redness or swelling and results from scintigraphy.
| Patient history | -Previous septic joint revisions -Previous infection -Detected microorganism in an external hospital during revision or diagnostic aspiration |
| Clinical appearance | -Present cardinal signs of inflammation (Pain, heat, redness, swelling, loss of function) -Elevated body temperature above 37.5°C |
| Diagnostic findings | -Elevated serum CRP and WBC -Detected microorganism in diagnostic joint aspiration -Fluid WBC above 1760 cells/µl in cases of assumed infection at least 6 weeks after primary TKA and above 10700 cells/µl within 6 weeks after primary TKA -Fluid PMN% above 73% in cases of assumed infection at least 6 weeks after primary TKA and above 89% within 6 weeks after primary TKA -Positive leukocyte scintigraphy or evidence for infection in bone scintigraphy |
| Intraoperative appearance | -Draining sinus -Purulence -Suspect scarred tissue |
Detailed description of eight cases with persistent infection during second-stage procedure. In eight cases a microorganism was found during the second-stage procedure. The microorganism was Staphylococcus epidermidis in every case. The duration of antibiotic therapy after first-stage procedure was at least 6 weeks except for one case where intraoperative samples and histologic examination showed no signs of an infection. Consequently the time to second-stage procedure was reduced.
| Intraoperative Appearance | Microorganism of First-Stage Procedure | Antibiotic Therapy After First-Stage Procedure | Duration of Antibiotic Therapy | Duration Between First-Stage Procedure and Second-Stage Procedure | Microorganism of Second-Stage Procedure | Antibiotic Therapy After Second-Stage Procedure | Duration of Antibiotic Therapy | Further Development |
|---|---|---|---|---|---|---|---|---|
| Purulence | Staphylococcus aureus | Vancomycin/Rifampicin, later Rifampicin and Clindamycin | 10 weeks | 17 weeks | Staphylococcus epidermidis | Avalox | 6 weeks | No persistent signs of infection |
| Purulence | Staphylococcus capitis | Cefuroxim, later Rifampicin and Penicillin | 6 weeks | 17 weeks | Staphylococcus epidermidis | Rifampicin and Avalox | 2 weeks | No persistent signs of infection |
| Purulence | No verified microorganism but histologic signs of infection | Cefuroxim, later Rifampicin and Staphylex | 6 weeks | 12 weeks | Staphylococcus epidermidis | Vancomycin, later Linezolid | 4 weeks | No persistent signs of infection, but wound healing disorder |
| No manifest infection | Propr. acnes | Cefuroxim | 6 weeks | 16 weeks | Staphylococcus epidermidis | Rifampicin, Clindamycin | 6 weeks | No persistent signs of infection |
| Purulence | Propr. acnes | Clindamycin, Penicillin | 6 weeks | 8 weeks | Staphylococcus epidermidis | Vancomycin, later Linezolid | 6 weeks (2 weeks Linezolid) | No persistent signs of infection |
| No manifest infection | Propr. acnes | Vancomycin, Clindamycin, later Clindamycin allone | 6 weeks | 10 weeks | Staphylococcus epidermidis | Vancomycin, Rocephin, later Linezolid | 6 weeks (2 weeks Linezolid) | Persitent manifest infection with multiple revisions |
| Purulence | No verified microorganism, no histologic signs of infection | Vancomycin, Clindamycin | 2 weeks | 7 weeks | Staphylococcus epidermidis | Vancomycin and Rifampicin, later Rifampicin | 6 weeks | No persistent signs of infection |
| Purulence | Propr. acnes | Cefuroxim, later Clindamycin | 6 weeks | 20 weeks | Staphylococcus epidermidis | Vancomycin, later Linezolid | 6 weeks (2 weeks Linezolid) | No persistent signs of infection |
Treatment Algorithm
In cases of a verified or assumed PJI we performed a standard algorithm following the proposed algorithms of Parvizi et al. and Tsukayama et al. [2, 5]. During each operation samples from at least five localizations were collected for microbiologic and histologic evaluation. Intraoperative infection was confirmed one of the following criteria were met: at least in two of five microbiologic samples an infecting germ was verified, the histologic examination proved an infection, gross purulence was noted or there were evidence of a sinus tract. The histologic evaluation was performed according to the classification of Krenn and Morawitz and colleagues [17, 18]. After the first-stage the patient was started on Vancomycin and Clindamycin antibiotics until cultures returned with sensitivities. Then culture directed antibiotics were administered intravenously for two weeks followed by antibiotic treatment per os for a total of six weeks of treatment. Eight weeks after first-stage procedure, with at least a two-week antibiotic free interval, a diagnostic aspiration was performed. When the final results of the aspiration were negative and there were no other signs of ongoing infection the second-stage procedure was performed. If signs or symptoms of infection persisted we performed a second look operation with spacer exchange and re-debridement. A second course of antibiotic therapy was initiated based on the intraoperative and culture results for at least another six weeks.
We evaluated a successful surgical outcome based on the need for further antibiotic treatment or operations after the second-stage procedure. A successful clinical outcome was defined as a functioning prosthesis without wound healing disorders, no sinuses tracts or other clinical evidence of a persistent infection. Unsuccessful outcomes were defined as infect persistence or if an above knee amputation (AKA) was performed consecutively.
Factors Associated with Outcome
For statistical analysis we analyzed: age (Age > 60 vs ≤ 60), sex, previous operations, obesity (BMI > 30 vs ≤ 30), nicotine abuse, diabetes mellitus, compromised immune status (including glucocorticoid use), and chronic anticoagulant use. Additionally, we analyzed patients based on their Charlson comorbidity index score [19]. The presence of multidrug resistant germs or a mixed flora was analyzed as potential risk factor for failure.
Follow-Up Examination
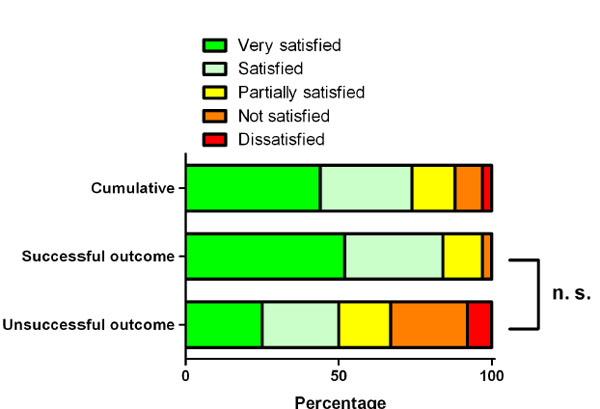
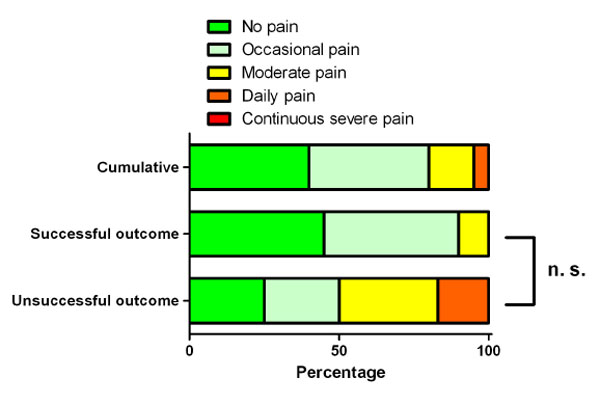
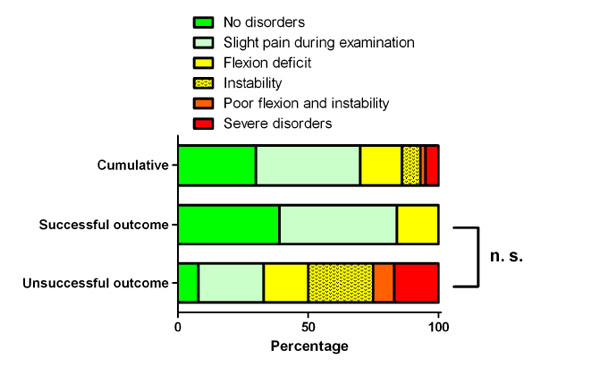
At a minimum of one year after second-stage procedure a follow-up examination was performed. The pain and satisfaction were evaluated into five categories and clinical examination into six categories. Satisfaction was categorized in very satisfied, satisfied, partially satisfied, not satisfied and dissatisfied. The patient’s pain was categorized into: no pain, occasional pain, moderate pain, daily pain or continuous severe pain. The patient’s clinical examination were categorized into: no abnormalities, slight pain during examination (but good flexion and no instability), flexion deficit, instability, flexion deficit and instability and severe abnormalities. A flexion of less than 90° was classified as a flexion deficit. Severe pain during the follow-up examination with minimal range of motion and gross instability was classified as a severe abnormality.
Statistical Analysis
The data collection and analysis was performed with Graph Prism 5 (GraphPad Software, Inc., La Jolla, CA 92037). Factors associated with outcome were analyzed using the chi-square test with Yates’ correction. In addition odds ratios were assessed. Odds ratios are illustrated with 95% Confidence interval (CI). Data on ordinal scale (satisfaction, pain, disorders) was analyzed using the Mann-Whitney-test. A p-value < 0.05 was deemed to be statistically relevant.
RESULTS
Our study consisted of 21 male and 29 female patients, with a mean age at first-stage procedure of 65.4 ± 10.6 years (mean ± standard deviation [SD]). The first-stage revision arthroplasty was performed 46.6 ± 39.0 month (mean ± SD) after primary TKA. 12 of 50 patients conformed to the definition of an unsuccessful treatment leading to a success rate of 76.0%.
During first-stage procedure the cultures were positive in 29 cases and in 21 cases no germ could be isolated. The germ isolates included: Staphylococcus epidermidis (eleven cases), Staphylococcus aureus (seven cases including three times MRSA), Proprionibacterium acnes (five cases), Streptococcus agalactiae (three times), two cases each of Enterococcus faecalis, Escherichia coli, Staphylococcus capitis and one case each of Micrococcus luteus, Bacillus species and Staphylococcus warneri. Five patients had a mixed flora. Additionally, three patients the histological examination was positive for infection but an infecting microorganism could not be isolated.
We used a mobile Refobacin cement spacer. For five cases additional Clindamycin was added, in one case Vancomycin and in one case Vancomycin and Clindamycin were added. The duration between first-stage procedure and second-stage procedure was 11.3 ± 5.6 weeks (mean ± SD). The duration of antibiotic therapy was 5.4 ± 2.2 weeks (mean ± SD). When bacteria were isolated the antibiotic therapy was culture directed. The diagnostic aspiration after first-stage procedure was negative in every case. However, two of the patients had gross purulence when performing the second-stage procedure. In these patients a lavage and spacer exchange was performed. The antibiotic therapy was resumed for an additional six weeks. During both revisions no microorganism could be verified and the second-stage procedure was performed again. For the other 48 patients bacteria was detected in eight patients during second-stage procedure. In each of these cases, a Staphylococcus epidermidis was found (Table 2). In five cases the germ isolated from the second surgery was different than the original isolate.
During second-stage procedure in three cases a septic arthrodesis was performed after the explanation of the antibiotic spacer. In one case the indication for arthrodesis was a persistent infection after three one-stage and an additional two-stage revision arthroplasty with persistent clinical sings of an infection and an elevated body temperature. Despite the clinical signs of infection during the first-stage procedure and the arthrodesis an infecting microorganism could not be grown in culture. Another arthrodesis was performed due to a persistent infection with delayed wound healing and a persistent sinus tract. This patient had Staphylococcus aureus isolated during the first-stage procedure and there were no sings of a infection during the arthrodesis. The indication for the third arthrodesis was a persistent infection after a one-stage revision arthroplasty. The intraoperative samples verified Staphylococcus epidermidis at the time of first-stage procedure. In all other cases a second-stage procedure of total knee prosthesis was performed.
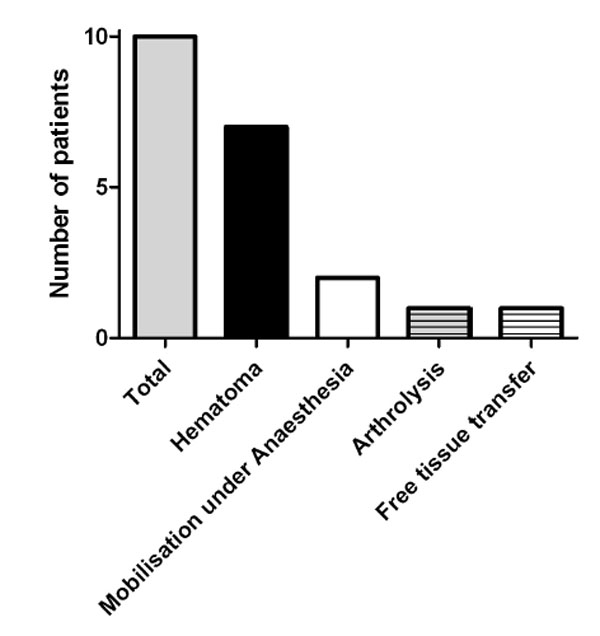
After first- and second-stage procedures a total of 20 revision procedures were necessary. The indications for the revisions are illustrated in Figs. (1, 2). Additionally two patients had a re-first-stage procedure and an arthrodesis performed for persistent infection. One patient had another two-stage revision arthroplasty and one patient had an AKA due to persistent infection. In one case a one-stage revision arthroplasty was performed because of an aseptic loosening. Two patients had two revisions resulting in 23 patients (46.0%) where further operations were necessary.
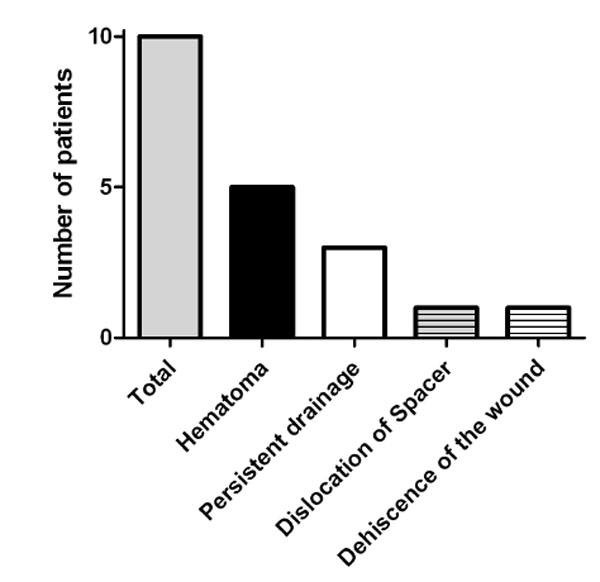 |
Fig. (1). Revisions after first-stage procedure of the prosthesis. The presence of hematoma was the most common reason for revision and persistent drainage was the second most common cause. |
Risk Factors for Poor Outcomes
Prior attempt at a revision arthroplasty after the primary TKA affected the success rate. 28.9% (11/38) of the successful group and 66.7% (8/12) of the unsuccessful group had previous revision arthroplasties (chi-squared 4.02; Odds ratio 4.9, 95% CI 1.2 to 19.7; p = 0.045). Furthermore, any additional operations after the primary TKA were found to have an effect. 36.8% (14/38) of the successful group and 75.0% (9/12) of the unsuccessful group had further operations after the primary TKA (chi-squared 3.92; Odds ratio 5.1, 95% CI 1.2 to 22.2; p = 0.048). Nicotine abuse was present in 36.8% (14/38) of the successful group and 75.0% (9/12) of the unsuccessful group (chi-squared 3.92; Odds ratio 5.1, 95% CI 1.2 to 22.2; p = 0.048). We noted that patients with a higher Charlson comorbidity index had a lower success rate. 42.1% (16/38) of the successful group and 83.3% (10/12) of the unsuccessful group had a Charlson comorbidity index above a value of 2 (chi-squared 4.67; Odds ratio 6.9, 95% CI 1.3 to 35.8; p = 0.031). The presence of a mixed flora was strongly associated with unsuccessful outcome. 0.0% (0/38) of the successful group and 41.7% (5/12) of the unsuccessful group had a mixed flora (chi-squared 13.27; Odds ratio 56.5, 95% CI 2.8 to 1134.0; p < 0.001). However, the presence of multidrug resistant organisms did not statistically significantly affect the success (chi-squared 3.53; Odds ratio 12.3, 95% CI 1.1 to 133.0; p = 0.06).
We did not found an effect on success rate for age (p = 0.809), sex (p = 0.38), rheumatoid arthritis (p = 0.277), body-mass index (p = 0.883), diabetes mellitus (p = 0.64), immune suppression (p = 0.745) and chronic anticoagulant use (p = 0.57).
Follow-Up Examination
A follow-up examination was performed at least one year after second-stage procedure of the prosthesis with 43 patients (86%). For the rest the data was incomplete or a further operation was needed. 19 patients were very satisfied and 13 satisfied. Five patients were not satisfied or dissatisfied. 17 patients had no pain. Another 17 patients described occasional pain and two patients indicated daily pain. At the follow-up examination a persistent flexion deficit of less than 90° was seen in seven cases, a persistent instability in three cases. One patient had a flexion deficit and instability and two patients had severe abnormalities. 13 patients had a clinically normal examination. When patients with successful outcome and patients with unsuccessful outcome were compared the unsuccessful outcome group had a lower number of satisfied patients and patients without pain and disorders. Furthermore all dissatisfied patients and patients with daily pain and severe disorders were in the unsuccessful outcome group. The differences between the groups were statistically not relevant (Figs. 3, 4 and 5).
DISCUSSION
Behind aseptic loosening and instability, PJI is the third leading reason for revision total joint arthroplasty [20]. The treatment of septic or aseptic complications after TKA varies considerably and this makes the accuracy of diagnosing of an ongoing infection critical. In addition, our clinical experience has shown that many patients underestimate the consequences of performing a two-stage revision arthroplasty. Therefore, the aim of this study was to evaluate the outcome of two-stage revision arthroplasty. We focused on factors that effected successful reimplantation.
A high accuracy of diagnostic a PJI is crucial to determine the most appropriate therapeutic pathway. Published data from Schindler and Kusuma query whether serum inflammatory markers and diagnostic aspiration is sufficient to confirm the diagnosis [8,10]. Alijanipour et al. also confirmed this assessment and they advised a higher threshold for CRP of 23.5 mg/l because it improves the diagnostic accuracy [4]. There are studies that support and refute the accuracy of diagnostic aspiration. WBC count of the aspirate should elevate the accuracy compared to fluid culture only [1, 21]. The sensitivity and negative predictive value are of great importance because the main interest of diagnostic is to exclude an infection. In verified or assumed cases of infection a two-stage revision is recomended.
The problem of a remaining diagnostic uncertainty is even increased in cases of assumed low-grade infection. Clinical examination and diagnostic parameters like serum inflammatory markers and diagnostic joint aspiration are commonly normal [22-24]. This is reflected in a high number of cases of assumed low-grade infection in the present study where an infecting microorganism could not be isolated. Another reasons for negative tissue samples in cases of assumed low-grade infection might be a low germ load or the biofilm [8]. Sonication of removed implants is discussed to improve the sensitivity of microbiological examinations via disrupting the biofilm and thereby increasing the number of germs isolated on culture [25]. As an additional diagnostic marker Interleukin-6 seems to be helpful. It had a high accuracy in cases of assumed low-grade infection [22-24]. These additional test were not performed in our study and may have affected our rates of microorganism isolation.
We found a success rate of 76.0%. That is nearly identical to a study from Tigani et al. who published a success rate 76.4% [14]. Still the published success rate after two-stage revision arthroplasty of the knee showed a high discrepancy and ranged from 37.1% to 100% [12, 14, 16, 26, 27]. There are several reasons for this discrepancy. First, the definition of a persistent infection is variable and thereby rate of success varies. Second, the diagnostic criteria and treatment differs between the studies. Third, the patient cohorts are heterogeneous.
Zmistowski et al. analyzed whether a persistent PJI is really is a persistent infection or whether it is a new infection. They described that only in about one third of the cases the same bacterium was found [28]. That gives evidence that further operations include a higher risk of further infections. However, Romano et al. showed that the two-stage revision arthroplasty leads to higher rate of eradication of an infection than the one-stage revision [12]. Tigani et al. highlighted the importance of the patient’s comorbidities on the outcome [14].
The rate of patients who received additional operations after first- and second-stage procedure was 46%. One potential reason for the high revision rate might be the case mix at a referral center for revision arthroplasty. A high number of patients had previous attempts at revision, including revision arthroplasties. Additionally, patients’ comorbidities in a referral center might be more severe when compared to other hospitals. Optimizing patients’ general health status, especially diabetes mellitus, prior to performing a revision arthroplasty can play a key component in the patient’s successful reimplantation [28]. Despite a high revision rate 32 patients were at least satisfied and 34 patients had no or just slight abnormalities.
The current study has limitations worth considering. The retrospective design leads to an incomplete picture of the outcome. We categorized the presence of pain and range of motion into several categories but the specific numbers for pain on a visual analog scale or the numerical range of motion may have been of interest. In addition, several different surgeons performed the surgeries on our cohort of patients and the transport of samples from the operation room to the department of microbiology was not standardized. Furthermore, the surgeons may have obtained samples from different locations and with different techniques. These points might have an impact on the results. This study does not have a control arm and a there were not any standardized outcome parameters. Additionally due to missing standardized preoperative data we were not able to estimate the improvement of the patients from preoperative to postoperative.
Treating PJI remains difficult, both for the surgeon and the patient. The data of this study could help to give more prognostic information to the patients regarding the likelihood of successful two-stage revision arthroplasties and the likelihood for additional procedures. This information might help to make a true informed consent for this procedure possible.
CONFLICT OF INTEREST
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
SUPPLEMENTARY MATERIAL
Supplementary material is available on the publisher’s web site along with the published article.
ACKNOWLEDGEMENTS
Declared none.