All published articles of this journal are available on ScienceDirect.
Comparison of Pre- and Postoperative Hemoglobin and Hematocrit Levels in Hip Arthroscopy
Abstract
Purpose :
to assess the loss in hematocrit and hemoglobin, if any, 24 hours after hip arthroscopy.
Methods :
thirty-five patients were included. Laboratory tests including complete blood count and white blood cells were performed one week prior to surgery and 24 hours after. Surgical time, volume of saline perfusion and pump perfusion was also recorded.
Results :
mean preoperative hematocrit was 42.01% (4.63 SD), whereas mean postoperative hematocrit at 24 h decreased to 36.78% (SD 5.11) (p <0.021.). Mean preoperative hemoglobin was 14.23 g/dL (1.73 SD), and mean postoperative hemoglobin at 24 h decreased to 12.40 g/dL (SD 1.92) (p =0.03.). Platelets and white blood cells, as well as the remaining biochemical parameters showed no significant difference between preoperative and postoperative samples. Lost blood volume worked out with the logarithmic method for estimated blood loss was which 0.78 liters (SD 0.45). Lost blood volume taking into account, the red blood cell mass was also 0.78 liters (SD 0.45).
Conclusion :
a significant decrease in hemoglobin and hematocrit after hip arthroscopy was observed. Although patients did not show clinical signs of anemia or bleeding, blood loss should be considered when planning a hip arthroscopy, especially in patients at risk of anemia. According to our results, we recommend a postoperative control analysis at 24 h.
Level of Evidence :
level II, Diagnostic Study.
INTRODUCTION
The performance of hip arthroscopy has increased exponentially worldwide due to the knowledge acquired of femoroacetabular impingement and is currently considered as an effective tool in the management of both extra and intra-articular pathologies [1-3].
Arthroscopy requires specific training and hip arthroscopy is particularly difficult due to the arduous access portals and the extensive learning curve that it entails. There are several publications that make reference to the complications that may arise within this technique, although most of them are temporary and easily avoided with preventive measures [4-8].
Both mechanical (nerve compression, labral and chondral lesions, breakage of material...) and medical complications (retroperitoneal hematomas, infections, venous thrombosis...) have been described [4-8]. A non-described complication involving this surgery is blood loss, which should be taken into consideration despite its low or even null clinical impact on patients. Moreover, blood loss is a surgical complication that should be especially aware of in at-risk patients such as those with cardiovascular or anemic problems. Although blood loss itself has been described in other orthopedic procedures, such as knee replacement or hip fractures [9-11], to our knowledge, the present manuscript is the first to describe its impact within hip arthroscopy.
The present work aims to evaluate the degree of blood loss, quantifying hemoglobin and hematocrit levels at 24 h post arthroscopic hip surgery, comparing the mentioned results with those obtained within one week before surgery.
Our hypothesis was that significant blood loss occurred following hip arthroscopy, described as a loss in hemoglobin and hematocrit values, as it has been described in other orthopedic procedures [9].
MATERIALS AND METHODS
A prospective study was performed between September and December 2012 with 35 consecutive patients undergoing hip arthroscopy. The inclusion criteria were patients older than 18 years, hip arthroscopy indication for femoroacetabular impingement (Cam, Pincer and mixed) and patients with hemoglobin (Hb) and hematocrit (Ht) levels within normal parameters (Hb 14-18 g/dL in men and 12-16 g/dL in women; Ht 40-54% in men and 37-49% in women). Excluded patients were those with blood dyscrasias, anemia, hematopoietic processes or under blood-related drugs (i.e. warfarin, acetylsalicylic acid). All patients included in the present study were informed that data concerning their case would be used for publication, and agreed to. Informed consent obtained in all cases and procedures were in accordance with the Helsinki Declaration of 1975 and revised in 2000.
All patients were clinically evaluated by the same surgeon, operated by the same surgical team, anesthetized by the same specialist with the same anesthetic technique (epidural with sedation). Surgery in all patients was a hip arthroscopy, first of all under leg traction to work at the joint and evaluate and treat any cartilaginous or labral lesion, Pincer-type deformities; and secondly, without traction, to treat Cam-type lesions with an osteoplasty with a motorized burr and radiofrequency for hemostatic control. Both the surgical indication and procedure were performed by an orthopedic surgeon with over 5-years of experience in hip arthroscopy and more than 100 hip arthroscopies performed per year.
Surgical times were recorded in all cases, as well as the volume of saline perfusion used during the arthroscopy and surgical infusion pump pressures.
Hemoglobin and hematocrit levels were recorded both preoperatively (one week before surgery) and 24 hours after surgery, also recording leukocytes and platelets in the same patients. Biochemical parameters in blood (urea, creatinine, triglycerides, magnesium, transaminases and bilirubin) were registered in order to record the absence of a blood dilution effect, during the same timings (one week before and 24 h after surgery).
In order to show data regarding lost blood volume two different methods were applied, previously studied by Biarnes et al. within blood loss in total knee arthroplasty [12]. Logarithmic analysis was applied upon estimated blood volume (which includes BMI and sex), taking into account preoperative and postoperative hematocrit. A second method was included evaluating the red blood cell mass. In the required cases, a blood transfusion can be performed if an important blood loss is observed. Transfusion requests are determined according to the hemodynamic stability, such as heart rate >120 bpm or absence of a blood pressure decrease >20 mmHg from a seating to a standing position [13]. The remaining parameters were evaluated to avoid the risk of a sample bias due to a possible blood dilution that could take place.
The following logarithmic equation was used to assess the volume of blood loss [12]:
VBL = EBL x ln (iHt /fHt)
VBL= volume of blood loss; EBL =estimated blood loss; iHt = initial hematocrit; fHt = final hematocrit.
The estimated blood loss (EBL) is worked out with an equation that considers sex, weight and height.
Men EBL (liters) = (0.0236 x height (cm)0.725) x (weight (Kg)0.425) - 1.229
Women EBL (liters) = (0.0248 x height (cm)0.725) x (weight (Kg)0.425) - 1.954
In order to make easier the following calculations, instead of estimating the volume of blood loss (VBL), the lost red blood cell mass (LRM) was calculated.
LRM = ERM x ln (iHt / fHt)
ERM = estimated red blood cell mass = EBL /iHt
The amount of red blood cell mass transfused, which is 200 cc for every packed red blood cells (RMT), must be added to the LRM, as well as the red blood cell mass retransfused (RMR) from the postoperative recovery, and thus obtaining the compensated lost red blood cell mass (cLRM).
cLRM = LRM + RMT + RMR
RMR = recovered blood volume / recovered Ht
To obtain the volume of blood loss (VBL):
VBL = EBL x cLRM / ERM
Statistical Analysis
The behavior of the abovementioned variables and its likeliness to a normal distribution was studied with Kolmogorov-Smirnov test. Student’s t-test for paired samples was used in all cases except for creatinine, where a non-parametric test was performed (Mann-Whitney U test).
Levene’s test was performed to assess the equality of variances. The Kolmogorov-Smirnov test showed normality of the distribution within all parameters except for postoperative creatinine. Thus, Student’s t-test for paired samples was used in all cases except for creatinine, where a non-parametric test was performed (Mann-Whitney U test).
Levene’s test observed non-significant results for all variables thus assuming equality of variances. Later, Student’s t-test for paired samples was used, observing significant differences between preoperative and postoperative hematocrit and hemoglobin values, being p value 0.021 and 0.030, respectively. The remaining parameters did not show significant differences. Mann-Whitney U test for creatinine was non-significant when comparing values before and after surgery (p value =0.748).
RESULTS
Thirty-five patients (20 males and 15 females) were included in the present study, undergoing surgery between September and December 2012. All patients followed controls, hip arthroscopy and physical therapy at our hospital. Mean age was of 43.37 years old (13.35 SD) and 12 left hips and 23 right hips were included.
The mean preoperative hematocrit was 42.01% (4.63 SD), whereas postoperative hematocrit at 24 h was 36.78% (SD 5.11), being this difference statistically significant (p =0.021.)
Regarding the preoperative hemoglobin, mean value was 14.23 g/dL (1.73 SD), which decreased to 12.40 g/dL (1.92 SD) 24 h postoperative, once more being this difference statistically significant (p=0.03). Figs. (1, 2) show a schematic representation of the hemoglobin and hematocrit values.

Schematic representation of the preoperative and 24-hour postoperative hematocrit values, showing a significant gap between them. Ht: hematocrit. Difference between both means was statistically significant (p=0.021), using Student’s t-test as the statistical test.
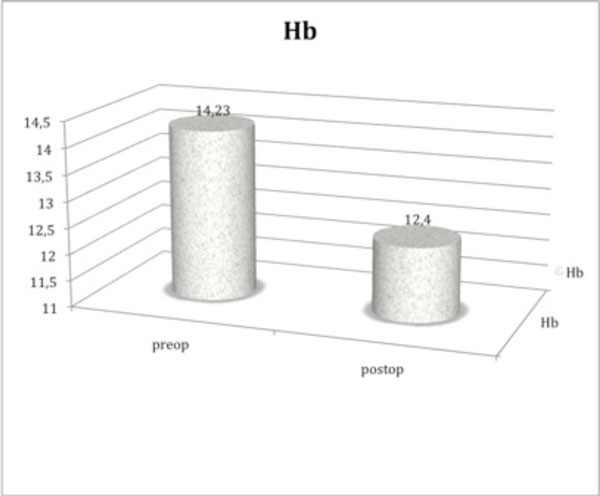
Schematic representation of the preoperative and 24-hour postoperative hemoglobin values, showing a significant gap between them. Hb: hemoglobin. Difference between both means was statistically significant (p=0.03), using Student’s t-test as the statistical test.
Variables studied for the present study, preoperative (pre) and postsurgery (post). Hematocrit and hemoglobin values were the only parameters to show statistically significant differences between the preoperative and postoperative results. The remaining measurements were non-significant. SD: standard deviation.
| Mean | SD | p-Value | ||
|---|---|---|---|---|
| Hematocrit (%) | pre | 42.02 | 4.64 | |
| post | 36.78 | 5.12 | 0.021 | |
| Hemoglobin (g/L) | pre | 140.24 | 10.74 | |
| post | 120.41 | 10.93 | 0.03 | |
| Platelet (x mm3) | pre | 233,646.36 | 42,954.1 | |
| post | 209,000 | 32,695.6 | 0.06 | |
| Leucocyte (x mL) | pre | 6427.27 | 1461.6 | |
| post | 6881.82 | 1362.2 | 0.083 | |
| Urea (mg/dL) | pre | 30.82 | 8 | |
| post | 27.82 | 7.77 | 0.05 | |
| Creatinine (mg/dL) | pre | 0.93 | 0.21 | |
| post | 1.02 | 0.3 | 0.748 | |
| Triglycerides (mmol/L) | pre | 0.83 | 0.29 | |
| post | 0.91 | 0.43 | 0.562 | |
| Aspartate transaminase (IU/L) | pre | 21.36 | 8.57 | |
| post | 21.55 | 8.44 | 0.811 | |
| Total Bilirubin (mg/dL) | pre | 0.59 | 0.18 | |
| post | 0.62 | 0.15 | 0.824 |
On the other hand, both the platelets and leukocytes levels showed no significant difference between preoperative and postoperative samples (233,636.36 platelets/μL (42,954.09 SD) vs 209,000 platelets/μL (32,695.56 SD), p >0.05 & 6427.27 leukocytes/μL (SD 1461.56) vs 6881.81 leukocytes/μL (1362.21 SD) p>0,05). The values of creatinine, triglycerides, magnesium, bilirubin do not show significant differences at the same timings, as it is described in Table 1.
Lost blood volume worked out with the logarithmic method for estimated blood loss which was 0.78 liters (SD 0.45). Lost blood volume taking into account in the red blood cell mass was also 0.78 liters (SD 0.45).
No active bleeding or anesthetic sensor anomalies occurred during any surgery. Neither was any active bleeding observed during postoperative hospitalization. No patient presented clinical symptoms of anemia (tachycardia, hypertension, pallor). Patients started walking with the aid of crutches at 24 hours after surgery with well-tolerated pain using analgesics (acetaminophen 1 gr/8 h iv) and anti-inflammatory (ibuprofen 600 mg/8 h) and were discharged from hospital 36 hours after surgery.
No patient received a blood transfusion in the present study, either during or after surgery.
DISCUSSION
The main finding of the present study was that after hip arthroscopy an average 0.78 liters of blood is lost, This newly described scenario can place in cardiovascular risk patients undergoing hip arthroscopy, due to almost a 2-point loss of hemoglobin and almost 6 of hematocrit.
Several studies describe complications within hip arthroscopy, however those related to perioperative bleeding in hip arthroscopy have yet not been described [4-8]. Complications associated with hip arthroscopy are firstly described due to traction and patient positioning, following chondral lesions during surgery at entry portals, extravasation of irrigation fluid, including abdominal cavity [14, 15], and other common alterations such as infection, deep vein thrombosis or breakages of surgical material [7]. The progression of avascular necrosis of the femoral head is more of a theoretical risk than a real one although the appearance of bone edema is more frequent [7]. Neurologic injuries, mostly transient, such as lateral femoral cutaneous neuropathy are the most frequent and represent a major part of the incidence of complications (0.5-6.4% of incidence) [6, 7].
Regarding hip surgery, both in fractures and arthroplasty, not only blood loss is a known for important complication, there are blood transfusion protocols and medications systematically performed in order to minimize perioperative bleeding [16, 17]. Over 50% of patients require the use of allogeneic blood transfusions [18] due to open surgery with wide surgical approaches, femoral osteotomy and reaming of the femoral canal and acetabular space.
Tourniquet is usually used in arthroscopic ACL reconstruction to minimize surgical bleeding [13]. Berg et al. performed a hematocrit control both before and 24 hours after retiring drainages, following arthroscopic ACL reconstruction. In the present study, due to the absence of drainages, evaluation was performed at 24 hours, in the same lines as the previous study. Berg et al. observed a 9-point hematocrit decrease with this procedure, unaffected by the use of an intraoperative tourniquet [13]. This data is in line with the present study’s results, probably relating to the trochleoplasty and tunnelization performed during ACL reconstruction with the osteoplasty in our hip arthroscopies.
Pape et al. published a similar study examining the role of trochleoplasty in ACL reconstruction. Both the hemoglobin and hematocrit values were evaluated within 24 hours after drainage withdrawal, obtaining 2 to 3-point hemoglobin decrease and 7 to 10-point hematocrit decrease, statistically significant, in line with the abovementioned study in the present study [19].
Assessment of bleeding during the first 24 hours is a fact that authors have already studied in relation with bleeding in knee surgery. Ares-Rodriguez et al. reported that the rate of bleeding in patients undergoing knee replacement, assessed through the amount of blood collected in their suction drains, was effective during the first 16 hours [9]. Beyond that time the bleeding recorded through their drains was negligible, recommending removal of drains in that time span. In the present study, the fact that patients were assessed by blood sample analysis at 24 hours would be in agreement with the statements made by Ares-Rodriguez et al., concluding that beyond that figure, the rate of bleeding is insignificant and thus reducing our blood samples in this period of time [20].
In the present study, a decrease in both hemoglobin levels and hematocrit was found, with almost two points in the case of hemoglobin and seven points in hematocrit. In contrast, in the same measurements, no statistically significant decreases were noted in platelet or leukocyte levels, or within the biochemical parameters measured, therefore dismissing any chance of blood dilution possibility. Furthermore, taking into account the 0.78 liters blood loss, we could think that a perioperative and postoperative bleeding is present.
Blood loss in the study by Berg et al. was quantified in 1-1.5 blood units, which equals 560-840 mL, in line with the present study and that from Pape et al. [13, 19].
Given the above results within blood measurements, the observation that there is a decrease in hemoglobin and hematocrit after performing hip arthroscopy should be taken into consideration, especially in patients at risk of anemia, such as women with metrorrhagia, dysmenorrhea in athletes, etc. According to our results, we recommend a postoperative control analysis at 24 h for better medical management of patients.
Some limitations of the present study must be taken into consideration when reviewing our manuscript. First, ours is a single-center study. In addition, unaccounted perioperative blood loss can alter the perception of actual bleeding. Hematoma formation in the area surrounding the hip, joint bleeding, including the redistribution of fluids from serum infusion, could alter actual figures obtained. The lack of change in the numbers of platelets and leukocytes, and especially within the biochemical parameters evaluated, leave the latter possibility more unlikely, giving greater credibility to the data obtained.
Despite performing an exhaustive search of references, no more than the already cited in the present study have been found, regarding bleeding and hip arthroscopy.
Hip arthroscopy is a blood loss-procedure, which despite the absence of clinical signs could be potentially dangerous in patients with underlying anemic or blood disorders, given the actual outcomes. Therefore, this should be taken into account when planning the surgical procedure in patients with preoperative levels below safety margin limits.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Authors would like to acknowledge Mr. Thomas M. Oxlee for the revision and English translation of the present manuscript.


