All published articles of this journal are available on ScienceDirect.
Multiple Infectious Complications in a Severely Injured Patient with Single Nucleotide Polymorphisms in Important Innate Immune Response Genes
Abstract
Trauma is a major public health problem worldwide. Infectious complications, sepsis, and multiple organ dysfunction syndrome (MODS) remain important causes for morbidity and mortality in patients who survive the initial trauma. There is increasing evidence for the role of genetic variation in the innate immune system on infectious complications in severe trauma patients. We describe a trauma patient with multiple infectious complications caused by multiple micro-organisms leading to prolonged hospital stay with numerous treatments. This patient had multiple single nucleotide polymorphisms (SNPs) in the MBL2, MASP2, FCN2 and TLR2 genes, most likely contributing to increased susceptibility and severity of infectious disease.
INTRODUCTION
Trauma is a major public health problem worldwide, ranking as the fourth leading cause of death. In 2010, there were 5.1 million deaths from injuries and the total number of deaths from injuries was greater than the number of deaths from HIV/AIDS, tuberculosis, and malaria combined (3.8 million) [1, 2]. Infectious complications, sepsis and multiple organ dysfunction syndrome (MODS) remain important causes for morbidity and mortality in patients who survive the initial trauma [3, 4]. These complications increase the burden of cost to society.
There is increasing evidence for the role of genetic variation in the innate immune system on infectious complications in sepsis and trauma [5-10]. We describe a trauma patient with multiple infectious complications caused by multiple micro-organisms leading to prolonged hospital stay with numerous treatments. This patient had multiple single nucleotide polymorphisms (SNPs) in the innate immune system, most likely contributing to increased susceptibility and severity of infectious disease.
SINGLE NUCLEOTIDE POLYMORPHISMS
Humans have 23 pairs of chromosomes and, on average, all humans are 99.9% similar to any other human in terms of DNA sequence. The remaining 0.1% account for all the differences between humans These differences are known as ‘polymorphisms’. The coding regions of DNA contain the approximately 20,000 human protein-coding genes. The coding regions take up less than 2% of all DNA. More than 98% of the human genome is composed of non-coding DNA of which the function is partly unknown. Human diploid cells contain around six billion base pairs. These base pairs are pairs of nucleotides, the building blocks of DNA (A, C, T, and G).
Single Nucleotide Polymorphisms (SNPs; pronounced ‘snip’, plural ‘snips’) are variations of only one nucleotide in the sequence of DNA. Around 90% of all DNA variation is caused by SNPs making them the most common type of sequence variation. To date more than 60 million SNPs have been discovered in the human genome.
SNPs in coding regions of DNA may have the potential to alter the amino acid sequence in a protein but as a result of degeneracy this is not always the case; some proteins are coded by more than one codon. SNPs in coding regions are called synonymous if they do not affect the amino acid sequence and non-synonymous if they do influence the amino acid sequence of a protein. The non-synonymous SNPs can be divided into missense SNPs and nonsense SNPs. Missense SNPs result in the transcription of a different amino-acid, changing the functionality of the resulting protein as is the case in Factor V Leiden thrombophilia [11] and sickle cell disease [12]. A nonsense SNP results in the formation of a premature stopcodon leading to a truncated, incomplete protein as is the case in β-thalassemia in Sardinia [13] and some forms of cystic fibrosis [14].
CASE REPORT
Patient A, a 57 year old mechanic with a medical history of occasional use of cocaine and of gradually worsening vision in the last five months as a result of optic nerve atrophy tripped over a low brick wall at work and fell about one meter on the back of his head. He was immediately found to be tetraplegic by the paramedics and was transferred to a level 1 trauma center. Clinically, the complete cord lesion was found to be on the level of C3 and C4. Computed tomography showed a congenital narrowing of the spinal canal at the level of C3 and C4 as well as an old fracture of the third thoracic vertebra (Fig. 1). Magnetic resonance scanning showed hemorrhage in the myelum at the level of C3 and C4 as the reason for the tetraplegia (Fig. 2).
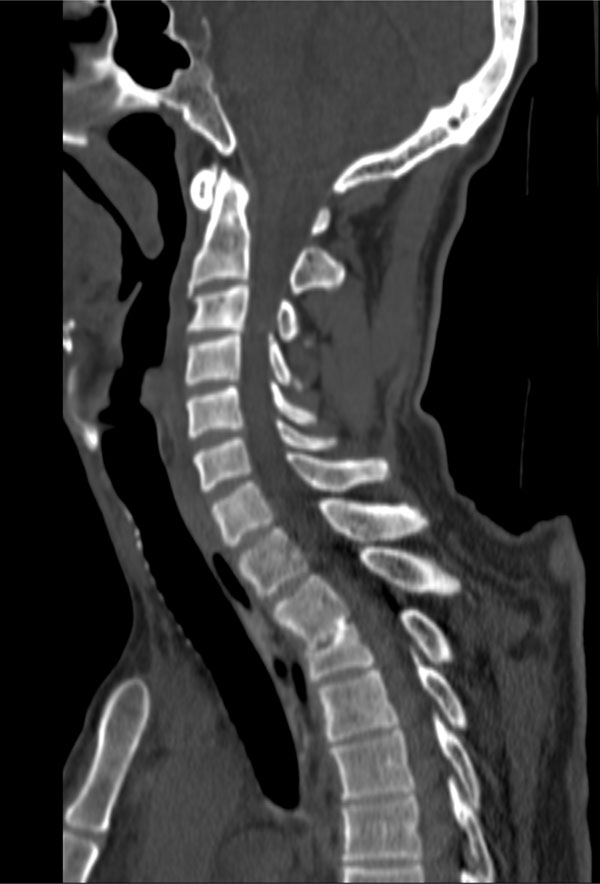
Computed tomography of discussed patient showing the congenital narrowing of the spinal canal at the level of C3 and C4. Also, a preexisting injury at the level of T2 and T3 can be seen.
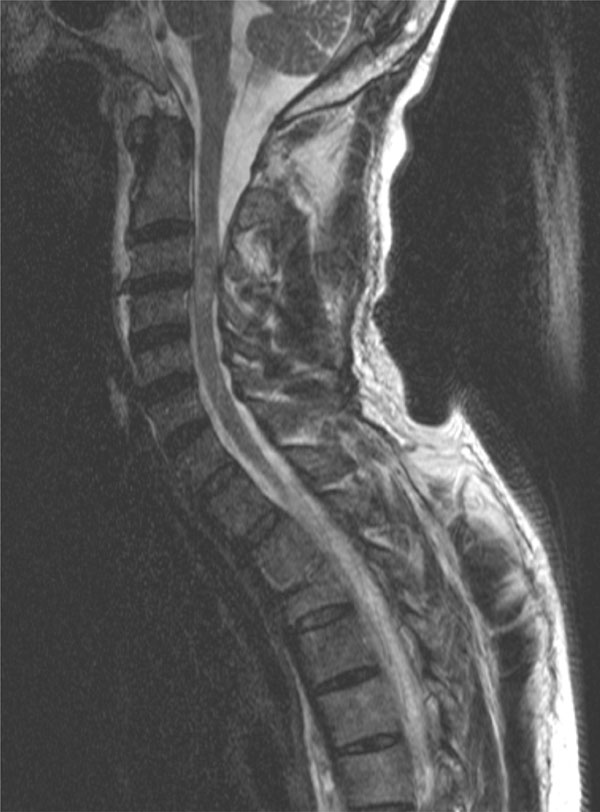
Magnetic resonance scanning of discussed patient shows bleeding in the myelum at the level of C3 and C4 as the cause for his complete cord syndrome.
Genotypes found in presented patient.
| Gene | SNP | OMIM | Cytogenic Location | dbSNP ID | Genotype of Our Patient |
|---|---|---|---|---|---|
| MBL2 exon 1 | codon 57 [C allele] | 154545 | 10q21 | rs1800451 | AC |
| MBL2 promoter | Y-221X | 154545 | 10q21 | rs7096206 | YX |
| MASP2 | Y371D | 605102 | 1p36 | rs12711521 | DD |
| FCN2 | A258S | 601624 | 9q34 | rs7851696 | AS |
| TLR2 | T-16934A | 603028 | 4q31 | rs4696480 | AA |
SNP: single nucleotide polymorphism. OMIM: Online Mendelian Inheritance in Man, an online catalog of human genes and genetic disorders. dbSNP ID: Single Nucleotide Polymorphism Database, a free public archive for genetic variation hosted by the National Center for Biotechnology Information [NCBI]
He was transferred to the Intensive Care Unit for ventilator support. On day 3 he developed acute respiratory distress syndrome (ARDS) and pneumonia in the right lower lobe, possibly as a result of aspiration, from which purulent sputum was removed repeatedly. The sputum grew Haemo-lytic Streptococcus group C, Streptococcus pneumoniae, Haemophilus influenzae and Enterobacter cloacae for which he was treated with piperacillin/tazobactam. On day 10 a percutaneous tracheostomy was used and weaning was possible. On day 20 patient was no longer dependent on ventilator support. He spent a total of 32 days on the Intensive Care Unit and 76 days in the hospital before being discharged to a rehabilitation center. After three months he was admitted again, this time to the department of Internal Medicine, for fever, diarrhea and productive cough. Radiology was suspect for pulmonary tuberculosis and Mycobacterium tuberculosis was eventually found in gastric contents. He was placed on tuberculostatic triple therapy. His diarrhea was explained by pseudomembranous colitis caused by Clostridium toxins. He was given metronidazol and was free of diarrhea after ten days. He developed deep venous thrombosis in the right subclavian vein ultimately leading to erysipelas and ulceration on the fingers from which Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Morganella morganii and Proteus mirabilis were cultured. The urine was positive for Klebsiella pneumoniae and Enterococcus faecalis. The ulcers were treated surgically.
Because this patient participated in a scientific trial studying genetic variation in trauma patients, his genome was sequenced for several single nucleotide polymorphisms (SNPs). Several SNPs were found: heterozygosity in MBL2 exon 1 (i.e., AC genotype), heterozygosity in MBL2 promoter region Y-221X, (YX genotype) homozygosity for the minor allele in MASP2 Y371D (DD genotype), heterozygosity in FCN2 T236M (TM genotype), heterozygosity in FCN2 A258S (AS genotype), homozygosity for the minor allele in TLR2 T-16934A (AA genotype), and heterozygosity in CD14 C-159T (CD genotype).
DISCUSSION
Mortality as a result of sepsis in the third peak of Trunkey [15] has not changed in recent times despite the improvements in treatments in the Intensive Care Unit leading to reduction in the incidence of sepsis itself [4]. The onset of sepsis in trauma patients is of course multifactorial, but genetic variation at the level of the innate immune system is certainly one important contributing factor. SNPs in genes coding for important proteins in the innate immune system, such as the complement system, may produce low serum levels of these proteins or they may leave these proteins dysfunctional. Hence, the immune response and cytokine response to trauma and infection is reduced leading to increased susceptibility and severity of infectious complications. These complications cause prolonged hospital stay and increase the use of antibiotics, the number of complications, and the cost of care to society.
The above presented patient was proband in a prospective study in severely injured trauma patients focusing on SNPs in the innate immune system and the influence on infectious complications. A number of SNPs were found in this patient (see Table 1).
In the lectin pathway of complement activation three important genes were studied: MBL2, MASP2, and FCN2. The MBL2 gene encodes for mannose-binding lectin (MBL), a protein that is secreted by the liver as part of the acute-phase response and is involved in innate immune defense. The ligands for MBL are expressed by a wide variety of microorganisms, and binding of the protein leads to opsonisation of the pathogen as well as activation of the complement system. Genetic variation in this gene leads to a dramatic decrease in circulating serum MBL. Heterozygosity for variants in exon 1 (i.e., an A0 genotype) conferred an increased risk for wound colonization and infection in severely injured patients [6]. This had previously only been demonstrated in a murine model of burns [16]. Also, the YX promoter genotype increased the risk of fungal colonization and infection in trauma patients [6]. Presented patient carried an AC genotype in exon 1 and a YX genotype in the promoter region. MBL activates the complement pathway through mannan-binding lectin serine protease 2 (MASP2). MASP2 Y371D DD homozygosity significantly increased the risk for SIRS and septic shock in trauma patients [6]. Moreover, a trend was noted for an increased risk of Gram-positive infections in patients with MASP2 Y371D DD genotype. Above presented patient carried the MASP2 Y371D DD genotype. FCN2 encodes for Ficolin-2, previously termed L-ficolin, a protein which is mainly produced in the liver and has been shown to have carbohydrate binding and opsonic properties in the innate immune system. The homozygous FCN2 A258S AS genotype increased the risk for developing septic shock in trauma patients [6]. Also, wound colonization and infection were significantly increased. A trend was noted for Gram-negative infections. In this article presented patient carried the FCN2 A258S AS genotype.
Deficiencies in the lectin pathway have been linked to susceptibility of various pathogens, for example MASP deficient mice are highly susceptible to Streptococcus pneumoniae [17] and also children [18] and adults [19] have previously been shown to be highly susceptible to S. pneumoniae with deficiencies in MBL and MASP2. In children lectin pathway deficiencies may play a role in Haemophilus influenzae [20] but this was not found in a cohort of adults with community acquired pneumonia [21]. The effect of MBL genotype on the susceptibility to Mycobacterium tuberculosis is controversial [22, 23]. In burn injury patients [16] and cystic fibrosis patients [24] MBL deficiency plays an important role on susceptibility to Pseudomonas aeruginosa infection and colonization. The FCN2 A258S polymorphism was previously shown to influence susceptibility to leprosy [25], influence colonization with Pseudomonas aeruginosa in cystic fibrosis patients [24] and influence renal transplant outcome [26].
As a membrane surface receptor, TLR-2 recognizes many bacterial, fungal, viral, and certain endogenous substances. The TLR2 T-16934A polymorphism was previously linked to spontaneous bacterial peritonitis in liver cirrhosis patients [27], atopic dermatitis, asthma and wheezing [28-30] and sarcoidosis [31]. The TLR2 T-16934A genotype was studied in a trauma population by one author [7] who found that the TLR2 T-16934A TA genotype increased the risk of a Gram-positive infection and SIRS. The TLR2 T-16934A AA genotype seemed to protect against urinary infection, oddly. However, patient A carried the TLR2 T-16934A AA genotype but developed positive urine cultures.
Infectious complications are multifactorial in origin. SNPs in the innate immune system contribute to susceptibility and severity of these infections and lead to prolonged hospital stay and increased cost. The presented patient demonstrates the clinical course of such complications that will be recognized by all surgeons. In the future we expect that initial genotyping will become routine workup in all trauma patients to quantify the individual risk for developing infections. Patients identified to be at risk for developing infectious complications can be prophylactically treated with antibiotics in an early stage or can be supplemented with plasma from mixed donors containing the deficient proteins. substitution therapy with purified or recombinant proteins has also produced clinical results, for example in the case of MBL-deficiency [32-36]. Further studies are needed in order to determine which genes affect this risk and to quantify their effect.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGeMENTS
Declared none.


