All published articles of this journal are available on ScienceDirect.
Assessment of Dosing and Patient Factors on the Efficacy of Warfarin Following Total Joint Replacement
Abstract
The purpose of this study was to determine the percentage of patients discharged with a subtherapeutic INR <1.8 using our institutions inpatient warfarin dosing nomogram following total joint arthroplasty (TJA). We examined predisposing risk factors for a subtherapeutic discharge (INR <1.8), including increased body weight, age, gender, end stage renal disease (ESRD), smoking, and peri-operative transfusion.
Chart review identified 249 patients for study inclusion. Logistic regression (LR) was used to identify associated risk factors for a subtherapeutic INR (<1.8) on day of discharge.
The majority of patients (58.6%, 146 of 249) following TJA surgery were found to have a subtherapeutic INR level (INR<1.8) at discharge (mean length of stay 2.6 days). Multivariate LR analysis found that weight greater than 180 lbs. (OR 2.08, CI 1.09, 3.98, P=0.027) was found to increase the odds of a subtherapeutic INR on day of discharge. Our results were not significant for weight 20% beyond ideal body weight, age (>65y), gender, peri-operative transfusion, smoking, ESRD or autoimmune disease.
A patient’s body weight influences response to warfarin following TJA. An inpatient warfarin dosing nomogram that takes into account a patient’s weight should be used to reduce the risk of subtherapeutic INR levels in obese TJA patients.
INTRODUCTION
Venous thromboembolism (VTE) is a major complication of lower extremity total joint arthroplasty (TJA), and reaching therapeutic levels of anticoagulation is an important component of post-operative care. In the absence of prophylactic anticoagulation, the incidence of venographic evidence of deep venous thrombosis (DVT) or pulmonary embolism (PE) approaches 40-60% following total joint replacement [1], with clinical evidence of DVT/PE reported around 2-3% following joint replacement. Several pharmacologic methods of anticoagulation exist, including but not limited to low molecular weight heparin (LMWH), unfractioned heparin, dabigitran, apixaban, rivaroxaban, aspirin, fondaparinux, and warfarin [2].
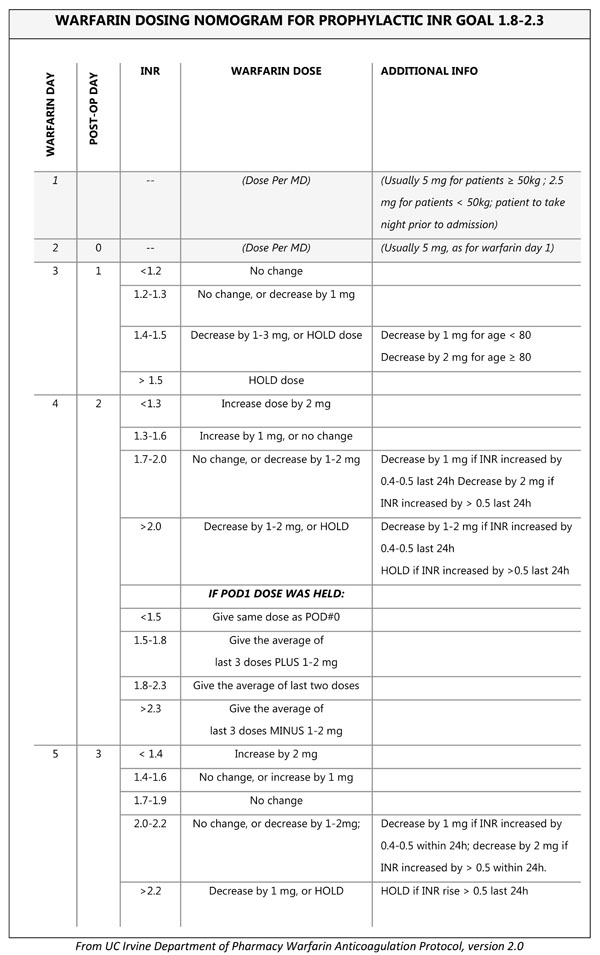
Our institution currently uses warfarin, dosed by a nomogram (Appendix 1), as well as mechanical sequential compression devices following TJA, with a target INR between 1.8 and 2.3. Warfarin has been used as a low cost anticoagulant since the 1950s [3], and is still a widely used method of anticoagulation following major orthopedic surgery [2, 4]. However, it has its limitations, including increased risk of bleeding, need for INR monitoring, variable dosing, and delay in reaching therapeutic effect. The mean time to therapeutic INR for a patient taking warfarin following TJA has been reported between 10 to 13 days [5, 6]. Given that the mean length of stay following total joint replacement in North America is between 2 to 3 days, and is constantly decreasing [7], it is expected that many patients will be discharged home with a sub-therapeutic INR. Aynardi et al. reported that 80% (352/441) of patients were discharged with a subtherapeutic INR (<2.0) [7]. However, part of a patient’s care while on warfarin includes INR monitoring in the outpatient setting, and it is anticipated that many of these patients will reach therapeutic INR levels by their first follow-up appointment.
Due to the short length of hospital stay and risk of DVT/PE after TJA, identification of risk factors for a subtherapeutic INR while on warfarin may aid in optimizing post-operative treatment. Warfarin works by inhibiting vitamin K epoxide reductase and reducing vitamin K dependent clotting factors II, VII, IX, and X [3]. Several factors have the potential to influence the efficacy of warfarin, including patient demographics, comorbid disease, and drug consumption. Patient demographics that have been indicated to influence INR response to warfarin are gender [8, 9], age [8, 10], and body weight [9]. Studies have shown that increasing age is correlated with an increased sensitivity to warfarin, and a lower maintenance dose of the drug is required to reach a therapeutic range [8, 10]. Therefore, it can be concluded that the reverse may be true, and that younger patients are more likely to be subtherapeutic compared to older patients. Male gender and increasing body weight have been associated with a higher maintenance dose of warfarin8 and a decreased likelihood to reach therapeutic INR levels during the short hospital stay following TJA [9]. In particular, patients weighing greater than 180 lbs. had a significantly higher portion of subtherapeutic INR patients compared to patients less than 180 lbs [9].
Click here to add question or purpose 1. The purpose of this study was to determine the percentage of patients discharged with a subtherapeutic INR<1.8 using our institutions inpatient warfarin dosing nomogram following total joint arthroplasty (TJA). Among patients who were discharged subtherapeutic (INR<1.8), we looked for predisposing risk factors, including increased body weight, age, gender, renal disease, smoking, and peri-operative transfusion.
METHODS
The study was approved by our institutional review board (IRB). The study retrospectively collected data using systematic chart review to identify patients undergoing elective total joint replacement, and treated throughout their hospital stay with warfarin. To be included in the study, patients had to undergo scheduled elective procedures to ensure they were treated with warfarin beginning the night before surgery per our institutions nomogram. Emergent patients that required surgery before initiation of warfarin the night before were therefore excluded. Patient’s who were already on anticoagulation with warfarin for other comorbid conditions were excluded from the study. Additionally, patient’s who were switched to an alternative anticoagulation agent greater than 1 day prior to discharge were excluded, both because they were no longer treated by our nomogram, but also because INR data was no longer available after the patient’s medication was switched. Initially, 306 patients were identified as being scheduled for TJA of the knee or hip between October 2012 and January 2014 (Fig. 1). Four cases were cancelled prior to surgery. Another 18 patient’s were excluded because they were not elective TJA, but rather emergent or urgent surgeries for acute femoral neck fractures. Among the remaining 284 patients, 15 were identified as having already been treated with warfarin before surgery for other medical conditions and were excluded. Another 20 patients were excluded because they received an alternative to warfarin for anticoagulation (15 patients) throughout their hospital stay, or were switched from warfarin to an alternative therapy during their inpatient stay (5 patients) >1 day prior to discharge. The remaining 249 patients were included in the study analysis. Among these patient’s 108 of them were scheduled to follow-up with our institution’s Coumadin clinic for INR monitoring, with the remaining 141 scheduled to follow-up with out side facilities where we did not have access to their medical records. Among the 108 patients scheduled for follow-up at our institution, 87 patients actually showed up for their scheduled appointments for INR monitoring and had medical records available for review containing INR data. All 249 patients included in the study underwent chart review to collect weight, gender, age, comorbid disease, smoking status, length of hospital stay, daily INR levels, and daily warfarin doses, and current medications.
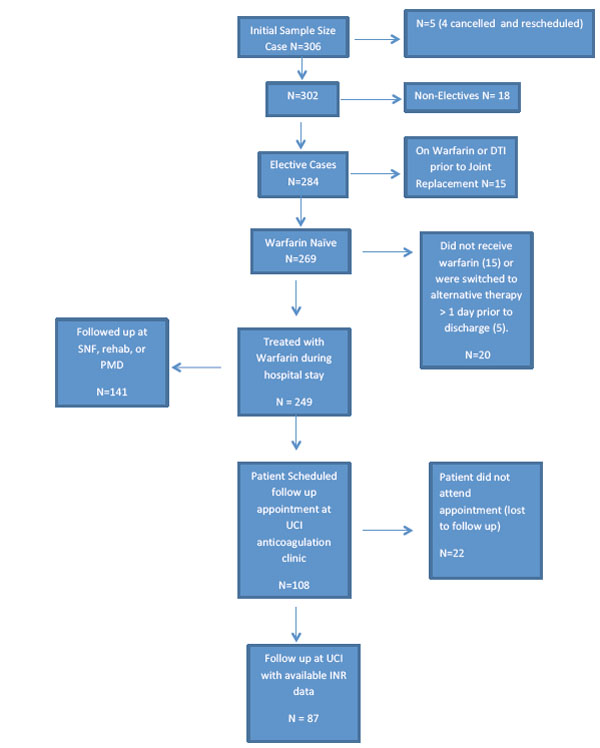
Flow Diagram. N=number of cases, DTI = direct thrombin inhibitor, Total in study: 249 cases.
Based on our nomogram (Appendix 1) we define subtherapeutic INR as <1.8, target therapy as INR between 1.8-2.3, and supratherapeutic INR >2.3. For statistical analysis, both target therapy and supratherapeutic, (i.e. INR ≥1.8) were considered as therapeutic.
Statistical Analysis
We determined the association between warfarin therapy characteristics (average daily dose, maximum daily dose, cumulative dose at discharge) and patient baseline factors’ effects on maintaining therapeutic INR (>1.8) at the time of discharge. Important baseline patient characteristics identified prior to data analysis includes age (≤65, >65), weighing 20% more than ideal body weight (IBW = 45.5 kg + 2.3 kg for each inch over 5 feet), weighing greater than 180 pounds, transfusion history, renal disease (CKD or ESRD), autoimmune disease, current smoker, and drug promoting warfarin. Our primary analysis is based on n=249 patients at discharge and our secondary analysis is n=86 patients with data at follow-up. Logistic regression models were used to assess the likelihood (odds) of subtherapeutic INR (<1.8) relative to therapeutic INR. Univariate logistic regression was used to assess simple contribution of each factor/covariates separately. This was followed by a multivariate logistic regression that included covariates with p-value ≤ 0.2 in the univariate analyses. Patient characteristics were summarized using standard summary statistics (mean, standard deviation, percent, frequencies). All tests were two-sided at significance level 0.05. Statistical analyses were performed using SAS, version 9.3 (SAS Institute, Inc.).
RESULTS
Patient Characteristics and Warfarin Dose
The study cohort had 249 patients with 103 (41.4%) therapeutic (INR≥1.8), including 39 (15.7%) supratherapeutic (INR≥2.4), and 146 (58.6%) subtherapeutic (INR<1.8) at discharge. Follow-up data assessing the efficacy (therapeutic/ subtherapeutic) at the first outpatient visit after discharge was available on 86 patients. Table 1 summarizes relevant baseline patient characteristics, length of stay (LOS) and warfarin dosing characteristics. The sample was 65% female, and the overall age range was 18-92, with 53% being younger than age 65; the average age of the study cohort was 63.7 (standard deviation [SD] 12.9). A majority of the study subjects have the following baseline risk factor profile: being greater than 180 pounds (55%), and greater than 20% of their ideal body weight (71%). Other patient characteristics include history of transfusion (14%), renal disease (4%), autoimmune disease (11%), current smoking (12%) status, and concurrently taking a drug that promotes effects of Warfarin (30%).
Summary of patient characteristics, warfarin dose and length of stay (n=249).
| Variable | Group | Count (Percent) |
|---|---|---|
| Sex | Male | 87 (35%) |
| Female | 162 (65%) | |
| Age Category | <65 | 133 (53%) |
| >=65 | 116 (47%) | |
| 20% greater than Ideal body weight (n=237) | yes | 178 (75%) |
| no | 59 (25%) | |
| Greater than 180 pounds (n=248) | yes | 136 (55%) |
| no | 112 (45%) | |
| Transfusion | yes | 35 (14%) |
| no | 214 (86%) | |
| Renal disease (CKD or ESRD) | yes | 11 (4%) |
| no | 238 (96%) | |
| Autoimmune Disease | yes | 28 (11%) |
| no | 221 (89%) | |
| Current Smoker | yes | 31 (12%) |
| no | 218 (88%) | |
| Drug Promoting Warfarin | yes | 75 (30%) |
| no | 174 (70%) | |
| Variable | Mean (SD) | Range |
| Age (yrs) | 63.7 (12.90) | 18-92 |
| Average Daily Dose as inpatient (mg) | 4.28 (1.16) | 1.6-7.5 |
| Maximum Daily Dose as inpatient (mg) | 5.39 (1.31) | 2.5-10.0 |
| Cumulative Dose by discharge (mg) | 14.30 (4.95) | 3.5-31.0 |
| Length of Stay (days) | 2.60 (0.97) | 1-13 |
SD = Standard Deviation; CKD = Chronic kidney disease; ESRD = End stage kidney disease.
Results of univariate logistic regression, modeling risk of subtheapeutic INR at discharge (n=249).
| Variable | Comparison | Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|
| Baseline Characteristics | ||||
| Sex | Male vs Female: | 1.45 | 0.85-2.47 | 0.18 |
| Age Category | < 65 vs >=65 | 1.71 | 1.03-2.84 | 0.039 |
| 20% greater than Ideal body weight | Yes vs No | 2.38 | 1.30-4.32 | 0.0047 |
| greater than 180 pounds | Yes vs No | 2.98 | 1.76-5.03 | <.0001 |
| Transfusion History | Yes vs No | 0.81 | 0.40-1.67 | 0.57 |
| Renal disease (CKD or ESRD) | Yes vs No | 0.39 | 0.11-1.36 | 0.14 |
| Autoimmune Disease | Yes vs No | 0.57 | 0.26-1.26 | 0.17 |
| Current Smoker | Yes vs No | 1.14 | 0.53-2.45 | 0.75 |
| Drug Promoting Warfarin | Yes vs No | 1.17 | 0.68-2.04 | 0.57 |
| Warfarin Dose and Length of Stay | ||||
| Average Daily Dose as inpatient (mg) | 3.99 | 2.82-5.65 | <.0001 | |
| Maximum Daily Dose as inpatient (mg) | 3.09 | 1.94-4.93 | <.0001 | |
| Cumulative Dose by discharge (mg) | 1.27 | 1.18-1.36 | <.0001 | |
| Length of Stay (days) | 0.45 | 0.30-0.68 | <.0001 | |
CI = Confidence interval.
Results of multivariate logistic regression with baseline characteristics only, modeling risk of subtherapeutic INR at discharge (n=249).
| Variable | Comparison | Adjusted Odds Ratio | 95% CI | P-Value |
|---|---|---|---|---|
| Sex | Male vs Female | 1.32 | 0.70-2.49 | 0.39 |
| Age Category | <65 vs >=65 | 1.41 | 0.80-2.49 | 0.23 |
| 20% greater than ideal body weight | Yes vs No | 1.70 | 0.84-3.42 | 0.14 |
| Greater than 180 pounds | Yes vs No | 2.08 | 1.09-3.98 | 0.027 |
| Renal disease (CKD or ESRD) | Yes vs No | 0.26 | 0.07-1.02 | 0.053 |
| Autoimmune Disease | Yes vs Notd> | 0.65 | 0.27-1.55 | 0.0.33 |
Multivariate model.
| (A) Multivariate model with average daily dose | ||||
|---|---|---|---|---|
| Variable | Comparison | Adjusted Odds Ratio | 95% CI | P-Value |
| Baseline Characteristics | ||||
| Sex | Male vs Female | 0.84 | 0.39-1.82 | 0.65 |
| Age Category | <65 vs >=65 | 0.99 | 0.50-1.96 | 0.97 |
| 20% greater than ideal body weight | Yes vs No | 1.07 | 0.44-2.56 | 0.89 |
| Greater than 180 pounds | Yes vs No | 1.67 | 0.76-3.68 | 0.20 |
| Renal disease (CKD or ESRD) | Yes vs No | 1.77 | 0.56-5.66 | 0.33 |
| Autoimmune Disease | Yes vs No | 1.26 | 0.26-6.00 | 0.78 |
| Warfarin Dose and Length of Stay | ||||
| Average Daily Dose as inpatient (mg) | 3.28 | 2.07-5.21 | <.0001 | |
| Maximum Daily Dose as inpatient (mg) | 1.48 | 0.81-2.72 | 0.20 | |
| Length of Stay (days) | 0.64 | 0.38-1.07 | 0.091 | |
| (B) Multivariate model with cumulative dose | ||||
| Variable | Comparison | Adjusted Odds Ratio | 95% CI | P-value |
| Baseline Characteristics | ||||
| Sex | Male vs Female | 0.85 | 0.40-1.84 | 0.69 |
| Age Category | <65 vs >=65 | 0.99 | 0.50-1.94 | 0.97 |
| 20% greater than ideal body weight | Yes vs No | 1.12 | 0.47-2.64 | 0.80 |
| Greater than 180 pounds | Yes vs No | 1.61 | 0.73-3.54 | 0.24 |
| Renal disease (CKD or ESRD) | Yes vs No | 1.85 | 0.58-5.91 | 0.30 |
| Autoimmune Disease | Yes vs No | 1.22 | 0.26-5.80 | 0.80 |
| Warfarin Dose and Length of Stay | ||||
| Cumulative Dose as inpatient | 1.29 | 1.16-1.42 | <.0001 | |
| Maximum Daily Dose as inpatient (mg) | 1.58 | 0.87-2.86 | 0.13 | |
| Length of Stay (days) | 0.25 | 0.14-0.44 | <.0001 | |
CI = Confidence interval.
Summary of patient’s characteristics, warfarin dose and length of stay for outpatient sample (n=87).
| Variable | Group | Count (Percent) |
|---|---|---|
| Sex | Male | 33 (38%) |
| Female | 53 (62%) | |
| Age Category | <=65 | 48 (56%) |
| >65 | 38 (44%) | |
| 20% greater than Ideal body weight (n=85) | yes | 67 (79%) |
| no | 18 (21%) | |
| greater than 180 pounds | yes | 48 (56%) |
| no | 38 (44%) | |
| Transfusion | yes | 8 (9%) |
| no | 78 (91%) | |
| Renal disease (CKD or ESRD) | yes | 5 (6%) |
| no | 81 (94%) | |
| Autoimmune Disease | yes | 7 (8%) |
| no | 79 (92%) | |
| Current Smoker | yes | 12 (14%) |
| no | 7474 (86%) | |
| Drug Promoting Warfarin | yestd> | 25 (29%) |
| no | 61 (71%) | |
| Variable | Mean (SD) | Range |
| Age (yrs) | 63.3 (12.4) | 18-88 |
| Average Daily Dose as inpatient (mg) | 4.50 (1.04) | 2.2-7.5 |
| Maximum Daily Dose as inpatient (mg) | 5.49 (1.24) | 2.5-10.0 |
| Cumulative Dose by discharge | 14.8 (4.57) | 6.0-26.0 |
| Length of Stay | 2.36 (.572) | 1-4 |
| Days to Follow-up | 6.27 (3.56) | 3-3-30 |
SD = Standard Deviation; CKD = Chronic kidney disease; ESRD = End stage kidney disease.
Patients available for follow up (n=87) data comparing inpatient (IP) discharge INR status to outpatient (OP) INR status at follow up.
| Status | OP Subtherapeutic | OP Target | OP Supratherapeutic | Total |
|---|---|---|---|---|
| IP Subtherapeuic | 28 | 16 | 15 | 59 |
| IP Target | 6 | 9 | 3 | 18 |
| IP Supratherapeutic | 6 | 1 | 3 | 10 |
| Total | 40 | 26 | 21 |
The average (SD) and range of average daily dose were 4.3 mg (SD 1.2); range 1.6-7.5). Average maximum daily dose was mean 5.4 mg (SD 1.3), with range 2.5-10 mg. The average cumulative dose received by a patient during their stay was 14.3 mg (SD 5.0) and range from 3.5 to 31 mg. The average LOS for all 249 participants was 2.60 (SD 0.97) days with all patients LOS between 1-6 days with the exception of one patient with a high LOS of 13 days. The analysis results reported below were not sensitive to inclusion or exclusion of this observation.
Warfarin Efficacy at Discharge
Results of the univariate logistic regression modeling the risk of having a subtherapeutic INR (relative to therapeutic INR) at the time of discharge are summarized in Table 2. Significant odds ratio (OR) over 1 indicate an increased risk of subtherapeutic INR, whereas values under 1 represent higher likelihood of achieving therapeutic INR. A significantly increased risk of being discharged with a subtherapeutic INR was associated with age younger than 65 years (OR 1.71, CI 1.03-2.84, p=0.039), having 20% higher than ideal body weight (OR 2.38, CI 1.30-4.32, p=0.0047), and weighing greater 180 pounds (OR 2.98, CI 1.76-5.03, p<.0001). Having a greater length of stay (OR 0.45, CI 0.30-0.68, p<.0001) was associated with a significantly lower risk of subtherapeutic INR.
To jointly examine the association of patient baseline risk factors with the risk of subtherapeutic INR at discharge, multivariate LR was used. Patient’s weighing >180 lbs. were at a greater risk of a subtherapeutic INR at discharge with an OR 2.08 (CI 1.09, 3.98, p=0.027). All other variables, including sex, age, body weight, renal disease, and autoimmune disease were not statistically significant (p-values >0.05) (Table 3).
To jointly examine the association of patient baseline factors, length of stay, and warfarin dosing characteristics with the risk of subtherapeutic INR at discharge, multivariate LR was used. The results from the multivariate LR including all covariates were similar to the multivariate LR regression including only covariates with univariate p-value of ≤ 0.2 (i.e., excluding transfusion history, current smoking status, and drug promoting warfarin). Also, because average daily dose and cumulative dose at discharge are highly collinear (r = 0.83, p < 0.0001); therefore, we present two final reduced models in Table 4 that include (A) average daily dose or (B) cumulative dose for their respective interpretations. These multivariate model results indicate that all patient characteristics and maximum daily dose were no longer associated with the risk of subtherapeutic INR at discharge (A) when average daily dose was accounted for; or (B) when LOS and cumulative dose were accounted for. Furthermore, based on these models, we found that the increase in OR for a 1 mg increase in average daily dose was 3.3 (95% CI 2.1-5.2, p<0.0001) and the increase in OR for 1 mg increase cumulative dose was 1.3 (95% CI 1.2 – 1.4, p<0.0001). Longer LOS was significantly associated with reduced risk of subtherapeutic INR at discharge in model (A) (OR=0.25, 95% CI 0.14 - 0.44, p<0.0001) and also showed trend in model (B) (OR=0.64, 95% CI 0.38 - 1.07, p=0.091).
Efficacy at Follow-up
We also analyzed limited available data on INR status at the first outpatient visit, for a subset of 87 participants (Table 5). In the follow-up sample, 40 patients (46.0%) had an INR in the subtherapeutic range and 47 patients (54.0%), including 21 (24.1%) supratherapeutic, were in the therapeutic range. The range of days between discharge and the follow-up appointment ranged from 3 to 30, with the average being 6.3 (SD 3.6) days. We found that all patient characteristics, warfarin dosing characteristics and days to follow-up were all not associated with the risk of subtherapeutic INR. We found that among the patients (n=59) with follow up data that were discharged subtherapeutic 31 patients reached an INR of 1.8 or greater at their first follow up (53%) (Table 6).
DISCUSSION
With a significant risk of venous thromboembolism (VTE) following TJA surgery, adequate anticoagulation must be achieved post-operatively. Warfarin is an effective, well known anticoagulant used for orthopedic patients. However, given the delayed effect of warfarin, and the relatively short post-operative hospital course following TJA, the identification of patients at risk of a subtherapeutic INR may improve patient morbidity and mortality.
Our data shows that 58.6% (146/249 patients) of patients were subtherapeutic at discharge following a mean hospital stay of 2.6 days (range 1-13 days). A study by Aynardi et al. had 79.8% of patients (352/441 patients) discharged at a subtherapeutic INR following a comparable mean length of stay of 2.8 days [7]. Our patients were started on warfarin the night before surgery; whereas Aynardi et al. began anticoagulation with warfarin the evening following surgery [7]. This may help explain why our data shows that 25.7% of patients (64/249 patients) were within target therapy range (INR 1.8-2.3) compared to 12.7% (56/441) in the study by Aynardi [7]. A major difference between the current study and the study by Aynardi et al. that likely contributes to the difference in findings is our range of target therapy was set slightly lower at an INR of 1.8 to 2.3 compared to 2.0 to 2.5 [7]. Indeed, if we classified our subjects based on the same criteria as Aynardi, the percentage of patients in subtherapeutic, target, and supratherapeutic range would be a comparable 76.3%, 12.9%, and 10.8%, respectively. Our follow-up data at the patient’s first INR monitoring appointment demonstrate that 46.5% of patients with available INR data (86 of 249 patients) were subtherapeutic at an average of 6.3 days after surgery.
This study shows that more than half of our patients undergoing TJA were discharged with a subtherapeutic INR, and just under half of that cohort were subtherapeutic nearly a week postoperative as well. Given the short length of stay to achieve INR goals following TJA, the slow onset of warfarin and long half-life, a strategy to incorporate the results of this study would be to identify patients with risk factors for a subtherapeutic discharge (i.e. increased body weight) and begin warfarin dosing earlier or at higher doses before and/or after surgery. Likewise, patients with renal disease may need to have preemptively reduced warfarin dosing to prevent overshoot of the narrow INR goal since we utilize a low-moderate intensity anticoagulation postop.
Based on our univariate LR, significant factors that increased the likelihood of a subtherapeutic INR were body weight and age less than 65 years. Previous studies have shown that body weight plays an important role influencing a patient’s response to warfarin. A study by Messieh et al. found that patients weighing more than 180 lbs. had a decreased warfarin response compared to patients weighing less than 180 lbs [9]. Additionally, these patients required a higher mean daily dose, and a higher maximum daily dose of warfarin during their hospital stay. They also were found to take longer to reach therapeutic INR levels after initiating warfarin therapy. Our univariate LR results are consistent with these findings, and show that a patient weighing greater than 180 lbs. was 3 times (p<0.0001) more likely to be discharged subtherapeutic than a patient weighing less than 180 lbs. With multivariate analysis of baseline characteristics, we found a more modest increased risk of 2.1 times (p=0.027) increased risk of subtherapeutic INR on day of discharge. Furthermore, patient’s who were categorized as obese based on a body weight exceeding 20% of their ideal body weight were likewise 2.4 times (p=0.0047) or 1.7 times (p=0.14) more likely to be discharged subtherapeutic, based on univariate and multivariate analysis respectively. However, only the univariate analysis showed statistical significance.
Increasing age, particularly greater than 80 years, has been shown to increase sensitivity to warfarin in multiple studies [8-10]. These studies demonstrated both a decreased average daily dose of warfarin and an increased risk of INR > 4 in patients greater than 80 years [8-10]. Based on these results we concluded that younger patients, defined as patient’s less than 65 years, would be at an increased risk of subtherapeutic INR levels at discharge. With univariate analysis we found that patient’s less than 65 years were 1.7 times (p = 0.039) more likely to be discharged subtherapeutic than those greater than 65 years. However, with multivariate analysis of baseline characteristics we found that although the odds ratio indicated an increased risk (1.4 times) of subtherapeutic INR it was not statistically significant (p=0.23).
In our study, several other factors believed to have an increased risk of a subtherapeutic INR, including male gender, smoking, and drugs promoting warfarin metabolism were not found to be significant. Although the direction of the odds ratios were in the expected direction (>1) none of these were statistically significant (p>0.05) in either the univariate or multivariate analysis.
Comorbid renal disease had been shown to potentiate the effect of warfarin in several studies [11-13]. The effect of comorbid chronic kidney disease (CKD) (GFR <60 ml/min) in patients treated with warfarin has been associated with a 25% reduction in the cumulative weekly dose compared to patients without CKD (GFR > 60 ml/min) [12]. In a study by Limdi et al., severe CKD (GFR<30 ml/min) was shown to have an incident rate of over anticoagulation (INR>4) nearly twice the incident rate of patients with moderate chronic kidney disease [13]. Furthermore, the incident rate of major hemorrhage in patients with severe CKD was 4 and 5 times more than patients with moderate or mild CKD respectively [13]. Taken as a whole these results indicate a potentiated response to warfarin in patients with comorbid renal disease. Although our study showed the same trend as these findings with an odds ratio of 0.39 (p=0.14) and 0.26 (p=0.053) by univariate and multivariate analysis respectively, these were not statistically significant.
Another factor initially believed to be associated with a decreased likelihood of subtherapeutic INR levels at time of discharge was allogenic blood transfusion during or shortly after surgery. In our study we included all patients that received a single unit of pRBC or more intraoperatively, or during their post-operative hospital stay. Although the results indicated a 1.2 fold reduction in the likelihood of a subtherapeutic INR level, the result was not found to be statistically significant (p=0.57) in the univariate analysis and was not even included for multivariate analysis. A previous study by Lenzini et al. showed there was a significant transient increase in INR levels following intraoperative blood loss [14]. We anticipated significant results in patient’s requiring transfusion because it not only indicated intraoperative blood loss, but also the lack of clotting factors in allogenic pRBC was believed to contribute to an elevated INR.
As length of hospital stay increases, a patient has a longer time to reach therapy and more days of warfarin treatment before discharge. Our results predictably showed that as length of stay increased the odds of a subtherapeutic INR at discharge decreased. An additional hospital day reduced the likelihood of a subtherapeutic discharge by 1.6 fold in the multivariate average daily dose and cumulative dose models.
There are several important limitations of the current observational study, including the relatively small number of patients, particularly available for follow-up data after discharge. As it is a single-site observational study, selection bias is a limitation. Other unmeasured confounders could change the estimated relationship of, for instance, average dose on subtherapeutic outcome. Additionally, genetics is known to play a role in warfarin response, and no genetic testing was done to control for genetic effects. Moreover, the effect of liver disease seems to play a significant role influencing response to warfarin, however, we did not have enough patients with comorbid liver disease to make any meaningful association in this study. Future data from multi-site observational studies affording larger sample size will help further elucidate the findings reported here.
Further research should be conducted to assess the clinical relevance of a subtherapeutic INR at discharge following TJA. Future data should be gathered to specifically look if there was a higher incidence of DVT/PE in patient’s who were discharged subtherapeutic remained subtherapeutic at their follow up appointments. In this study, the number of patients with follow up data after discharge was relatively small. As more data becomes available we remain interested in analyzing the effect of comorbidities and demographics, including body weight, on patients response to warfarin following discharge.
In conclusion, this study shows that our current dosing nomogram results in more than half (58.6%) of our patients being discharged at subtherapeutic levels. When looking at factors influencing warfarin response, body weight was the most important patient baseline risk factor following TJR. Patients weighing more than 180 lbs. were 2 times more likely to be discharged subtherapeutic. Finally, the results showing a longer hospital stay significantly decreased the likelihood of being discharged subtherapeutic, which may be of benefit to patients at risk of subtherapeutic INR levels. As a whole, the results of this study show that patients with an increased body weight are less likely to reach therapeutic INR levels using our current warfarin dosing nomogram.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
APPENDIX 1