All published articles of this journal are available on ScienceDirect.
Increase of Sodium Channels (Nav 1.8 and Nav 1.9) in Rat Dorsal Root Ganglion Neurons Exposed to Autologous Nucleus Pulposus
Abstract
Purpose:
It has been assumed that nucleus pulposus-induced activation of the dorsal root ganglion (DRG) may be related to an activation of sodium channels in the DRG neurons. In this study we assessed the expression of Nav 1.8 and Nav 1.9 following disc puncture.
Method:
Thirty female Sprague-Dawley rats were used. The L4/L5 disc was punctured by a needle (n=12) and compared to a sham group without disc puncture (n=12) and a naive group (n=6). At day 1 and 7, sections of the left L4 DRG were immunostained with anti-Nav 1.8 and Nav 1.9 antibodies.
Result:
At day 1 after surgery, both Nav 1.8-IR neurons and Nav 1.9-IR neurons were significantly increased in the disc puncture group compared to the sham and naive groups (p<0.05), but not at day 7.
Conclusion:
The findings in the present study demonstrate a neuronal mechanism that may be of importance in the pathophysiology of sciatic pain in disc herniation.
INTRODUCTION
Application of nucleus pulposus on nerve tissue has recently been linked to the occurrence of pain-related behavior in various experimental models [1, 2]. However, it seems likely that pain only appears, or is at least more pronounced, if exposure of the nerve tissue to nucleus pulposus is combined with mechanical deformation of the nerve tissue [1, 2]. This may suggest that nucleus pulposus sensitizes the nerve tissue to produce nerve root pain when the nerve tissue is deformed mechanically. There is also clinical evidence indicating that mechanical stimulation of a nerve root not previously exposed to nucleus pulposus results in only slight discomfort, whereas mechanical stimulation of a nerve root exposed to nucleus pulposus instead reproduces the sciatic pain [3]. Nucleus pulposus application on nerve tissue has also experimentally been found to induce electrophysiological changes such as activation of dorsal root ganglion (DRG) neurons [4] and ectopic discharge of the spinal dorsal horn neurons [5] as well as increased expression of sodium channels in neuronsin the DRG [6]. These data together suggest that nucleus pulposus induces hyperexcitability in DRG neurons.
Ion channels are very important in neuronal activity in generating and conducting action potential. Especially, sodium channels have been in focus in pain research, since the sodium channel blocker lidocaine is very useful clinically to block pain [7]. There are nine distinct voltage-gated sodium channel α subunits; Nav 1.1-1.9 [8]. Adult DRG sensory neurons may express a combination of Nav 1.1, Nav 1.6, Nav 1.7, Nav 1.8 and Nav 1.9 sodium channels [9, 10]. Nav 1.9 is mostly expressed in neurons with unmyelinated axons (C-fibers) [11, 12] and Nav 1.8 in neurons with unmyelinated and myelinated axons (A-fibers) [11, 13], and all these types of neurons are associated with transmission of nociceptive impulse. There are many reports about changes of these sodium channels after nerve injury and inflammation. However, it is unclear whether these sodium channels in the DRG neurons may be upregulated after lumbar disc herniation. The purpose of this study was to investigate the expression of Nav 1.8 and Nav 1.9 in DRG neurons exposed to nucleus pulposus using immunohisto-chemical methods.
MATERIALS AND METHODOLOGY
Thirty female Sprague-Dawley rats with an average body weight of 200-250g were housed in groups with free access to food (B&K Rat/mouse standard, BeeKay feeds & beddings, Sollentuna, Stockholm) and tap water. Temperature was kept at 21°C, light schedule was 12 hours daylight starting at 6:00 a.m. and 12 hours darkness starting at 6:00 p.m., and the humidity was kept at 50%. The experimental protocol was approved by the local animal research ethics committee.
Surgical protocol [1]: The rats were anesthetized by inhalation of isoflurane. Through a midline incision, the thoracolumbar fascia was incised just left to the spinous processes of the 4th and the 5th lumbar vertebrae. The erector spinae muscle was gently moved laterally to expose the left facet joint between the 4th and the 5th lumbar vertebrae. The joint, including articular processes, was carefully removed. By this procedure, the ligamentum flavum, the left L4 DRG and the intervertebral disc could easily be identified. To form a control group for the surgical exposure of the spinal canal, no additional procedures were undertaken in twelve rats (sham group: n=12). To perform a disc herniation, a 25 Gauge needle was used to puncture the exposed disc (NP group: n=12). By gently injecting some air through the needle there was a leakage of nucleus pulposus out to the DRG. To ensure contact between the nucleus pulposus and the nervous tissue the obtained nucleus pulposus was transferred with the tip of the needle to the 4th lumbar nerve root and DRG. No surgical intervention was performed in the Naïve group (n=6).
Immunohistochemistry
At day 1 and 7 after surgery, rats were deeply anaesthetized with an intraperitoneal injection of 0.2 ml pentobarbital (50mg/ml) and underwent intracardiac perfusion with 200ml saline followed by 200ml of Histofix (4% formalin)(Histolab Products AB, Sweden). The left L4 DRGs were removed and postfixed for 24 hours. The DRGs were dehydrated, embedded in paraffin, and cut into transverse 4.5μm sections. The sections were mounted on slides, deparaffinized with xylene, rehydrated, and processed immunohistochemically. The endogenous peroxidase in the sections was suppressed with hydrogen peroxidase (PeroxiDazed1) (Biocare Medical, Concord, CA, USA) for 5 min and then washed in Tris Buffered Saline (TBS) (Biocare Medical, Concord, CA, USA). For reducing non-specific binding, the sections were incubated with goat serum for 30 min at room temperature. The sections were then incubated with the primary antibodies, rabbit anti-Nav 1.8(Chemicon, Temecula, CA, USA), in a dilution of 1:100 or rabbit anti-Nav 1.9(Chemicon, Temecula, CA, USA), in a dilution of 1:200, for 30 min at room temperature. The incubated sections were washed in TBS and incubated with secondary antibody MACH2TM Polymer-HRP Conjugate (Goat Anti-Rabbit) (Biocare Medical, USA) for 30 min at room temperature. For visualizing the immunohistochemical staining, Betazoid DABChromagen Kit (Biocare Medical, USA) was used. Examination of the sections was performed with light microscopy. All analysis were performed in a blinded fashion.
Data Analysis
The total number of neurons and Nav -like immunoreactive (IR) neurons with visible nucleus in one complete transverse section was counted. Neurons showing higher intensity than background were considered positive using imaging analysis software (NIH image J). The cross sectional area of the neurons was also examined to categorize to small sized neurons (<600μm2), medium sized (600-1200μm2), and large sized neurons(>1200μm2). To be able to observe as many neurons as possible, we picked sections from the central one third of DRG as defined by the shape of the DRG. Six randomly selected sections were examined per rat. The percentage of Nav -IR neurons of the total number of neurons was calculated. The results are expressed as the percentage of Nav -IR neurons in the DRGs (mean ±standard error of the mean).
Statistical analysis was performed using ANOVA and Fisher’s PLSD. P-values less than 0.05 were considered significant.
RESULTS
Both Nav 1.8 and Nav 1.9 were stained positively in the somata of the DRG neurons in all experimental groups, especially in the small-sized neurons (<600μm2) (Figs. 1, 2).
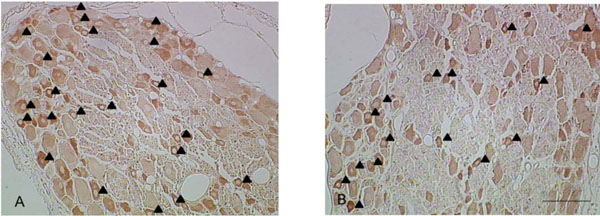
Representative pictures of Nav 1.8-IR neurons in the disc puncture group (A) and the sham group (B). Small and medium but not large sized neurons showed immune reactivity for Nav 1.8 (▲: Nav 1.8-IR neurons, Bar: 100µm).
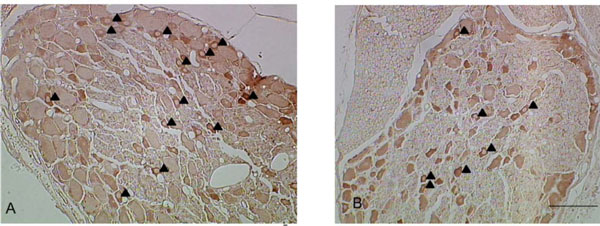
Representative pictures of Nav 1.9-IR neurons in the disc puncture model (A) and the sham group (B). Most of the Nav 1.9-IR neurons were small size neurons (▲: Nav 1.9-IR neurons, Bar: 100µm).
At 1st day after surgery, the percentages of Nav 1.8-IR neurons significantly increased in the disc puncture group compared with the Sham and Naïve groups in small sized neurons(p<0.05) (Fig. 3). There were no significant differences in medium and large sized neurons regarding Nav 1.8 expression. Nav 1.9-IR neurons in small sized neurons significantly increased in the disc puncture group compared with the naive groups (p<0.05) and there were also significant differences between sham and naïve group (p<0.05). There were no significant differences regarding Nav 1.9-IR neurons in the disc puncture group compared with sham group (p<0.1), (Fig. 4). There were also no significant differences in medium and large sized neurons. No significant differences were found among the three groups at 7 days after surgery in both Nav 1.8 and Nav 1.9-IR neurons (Figs. 3, 4).
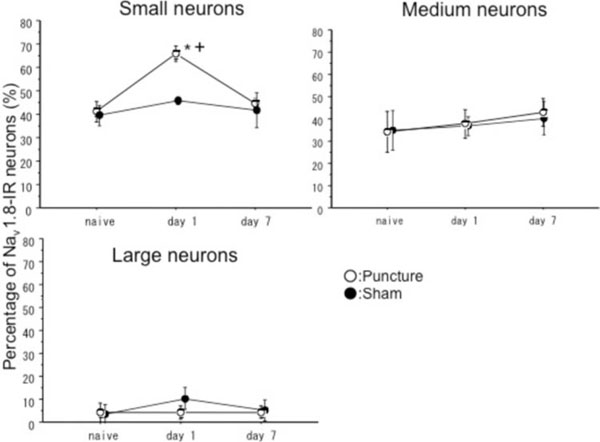
These graphs show percentages of Nav 1.8-IR neurons in small sized (<600µm2), medium sized (600-1200µm2) and large sized neurons (>1200µm2). There were significant differences between puncture and sham groups, and puncture and naïve groups in small neurons at Day 1. There were no significant differences in medium and large sized neurons. There were no significant differences among three groups at Day 7. * p<0.05 compared with naïve group, + p<0.05 compared sham group.

These graphs show percentages of Nav 1.9-IR neurons in small sized neurons (<600µm2), medium sized (600-1200µm2) and large sized neurons (>1200µm2). There were significant differences between puncture and naïve group, and sham and naïve group in small neurons at Day 1. The percentage of Nav 1.8-IR neurons tend to be higher in small sized neurons in puncture group compared with sham group at Day 1. There were no significant differences in medium and large sized neurons. There were no significant differences among three groups at Day 7. *p<0.05 compared with naïve group, # p<0.1 compared with sham group.
DISCUSSION
Leakage of nucleus pulposus from a lumbar disc to the dorsal root ganglion (DRG) induced transient increases of Nav 1.8 and Nav 1.9-IR neurons in the affected DRGs. In a corresponding model, pain-related behavior was only seen at one day postoperatively, but not 7 days after surgery [1]. It is known that nucleus pulposus can induce injury of axons [14] and inflammatory reactions around the nerve tissue [15]. Therefore, the transient increase in expression of Nav 1.8 and Nav 1.9 in the present study might theoretically have been influenced by neuronal damage as well as inflammatory responses, as induced by nucleus pulposus. Such changes of sodium channels in DRG neurons can be expected to be related to the electrophysiological properties of the neurons, including sensitization of DRG neurons. This could explain the pain mechanisms induced by nucleus pulposus. Nav 1.8 has been reported to be very important in pain sensation since a knock out of this channel [16] or blockage of this channel by specific blockers [17] showed a dramatic improvement of inflammatory pain in animals. A knock out of Nav 1.8 [18] and blockage of Nav 1.8 [17, 19, 20] has been found to also attenuate neuropathic pain. Neuropathic pain induced by partial axotomy induced a decrease of Nav 1.8 RNA and protein [21]. On the other hand, inflammatory pain induced by carrageenan caused an increase of Nav 1.8 [22]. Therefore, increase of Nav 1.8 induced by nucleus pulposus might follow the inflammatory effects of nucleus pulposus.
Nav 1.9 mRNA is likewise upregulated by inflammation and downregulated by axotomy [10]. Nav 1.9 null mice showed a decrease in inflammatory pain behavior but not in neuropathic pain [23]. Therefore, Nav 1.9 may be more closely related to inflammatory pain transmission than to neuropathic pain. Nav 1.9 current was increased by an inflammatory agent such as PGE2 [24]. Disc tissue harvested from the patients with sciatica contained many pro inflammatory mediators including PGE2 [25]. Therefore, in the model in the present study, there is a possibility that Nav 1.9 was increased by inflammatory reactions induced by nucleus pulposus. This, however, remains to be clarified in future studies.
One discrepancy between the present and previous studies was that upregulation was not found seven days after surgery in our study, whereas pain behavior changes, although less pronounced than at day 1 or 3, may still be seen at day seven [1, 2]. In the present study ion-channels were only studied in the dorsal root ganglion neurons and it is suspected that there may be a similar upregulation in the axons of the nerve roots that may sustain more than seven days. Also other modes of injury, such as simultaneous nucleus pulposus-exposure and mechanical deformation would be of certain interest, as well as the treatment with sodium channel-inhibitors. This will be assessed in future studies.
CONCLUSION
Clinically, sciatic pain induced by lumbar disc herniation is sometimes so pronounced that it is difficult to control the pain by presently available conservative treatment modalities such as NSAIDs, or nerve root block with local anesthetics and steroids. New strategies are needed to provide an alternative to surgery in such cases. Since the present immunohistochemical study indicates that sodium channels in DRG neurons may be increased by nucleus pulposus-exposure, specific blockages of neuronal sodium channels might offer such an alternative.
CONFLICT OF INTEREST
The author confirms that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
This work was supported by grants from the Swedish Research Council (521-2007-2956), the Gothenburg Medical Society, AFA Insurance, the Ollie and Elof Ericsson Foundation for Scientific Research, Stiftelsen Olle Engkvist byggmästare, the IngaBritt and Arne Lundberg Research Foundation, the Spine Society of Europe Task force on research and the Felix Neubergh Foundation.


