All published articles of this journal are available on ScienceDirect.
A 5-Year Follow-Up After Cartilage Repair in the Knee Using a Platelet-Rich Plasma-Immersed Polymer-Based Implant
Abstract
The aim of our study was to analyze the clinical outcome after repair of cartilage defects of the knee with subchondral drilling and resorbable polymer-based implants immersed with autologous platelet-rich plasma (PRP). Fifty-two patients with focal chondral defects were treated with subchondral drilling, followed by covering with a polyglycolic acid - hyaluronan (PGA-HA) implant (chondrotissue®) immersed with autologous PRP. At 5-year follow-up, patients’ situation was assessed using the Knee Injury and Osteoarthritis Outcome Score (KOOS) and compared to the pre-operative situation. The KOOS showed clinically meaningful and significant (p < 0.05) improvement in all subcategories compared to baseline. Subgroup analysis showed that there were no differences in the clinical outcome regarding defect size and localization as well as degenerative condition of the knee. Cartilage repair was complete in 20 out of 21 patients at 4-year follow-up as shown by magnetic resonance observation of cartilage repair tissue (MOCART) scoring. Covering of focal cartilage defects with the PGA-HA implant and PRP after bone marrow stimulation leads to a lasting improvement of the patients’ situation.
INTRODUCTION
Focal cartilage lesions of the knee are frequently found in symptomatic knees, do not heal spontaneously, are a major health problem and may progress to severe osteoarthritis [1].
The most common first-line treatment options for focal cartilage defects are bone marrow stimulating techniques like drilling or microfracturing [2-5]. These techniques activate subchondral mesenchymal progenitor or stem cells by introducing perforations into the subchondral bone, which allow for bleeding into the defect, followed by clot formation and in-growth of mesenchymal progenitor cells that form a fibrous to hyaline-like cartilage repair tissue [6, 7]. The clinical outcome of bone marrow stimulation is variable and may depend on the size and location of the lesion, the degenerative status of the knee, age, body mass index as well as the activity of the patient. This may lead to uncertain long-term functional improvements [8, 9]. However, compared to more advanced cartilage repair procedures like autologous chondrocyte implantation (ACI), the microfracture procedure is technically not demanding, cost effective, a relatively fast to perform one-step procedure and shows good short-term results in patients aged 40-45 and younger [3, 10, 11].
The cartilaginous repair tissue induced by bone marrow stimulation is hyaline-like to fibrous and is considered to be not durable. Therefore, developments emerged that aim at improving the microfracturing or bone marrow stimulating technique. These one-step cartilage repair approaches have in common that the microfractured defect is covered with a resorbable scaffold or membrane combined with blood derivatives like serum or platelet-rich plasma (PRP), leading to cell in-growth and subsequently to guided tissue repair [12]. The AMIC (autologous matrix-induced chondrogenesis) procedure uses a porcine collagen type I/III membrane to cover the microfractured defect that has been filled with fibrin glue and autologous serum or PRP [13-15]. Recently, chitosan-based BST-CarGel that is mixed with autologous whole blood and applied to microfractured cartilage lesions has been shown to result in greater defect filling and superior repair tissue quality after one year, compared to microfracture alone. However, the clinical outcome as assessed by Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) was significantly improved in both groups compared to baseline, but showed no differences between the treatment groups [16].
Our one-step cartilage repair approach relies on a mechanically resistant, synthetic, textile polyglycolic acid-hyaluronan (PGA-HA) implant that is used for the covering of microfractured cartilage defects. PGA-HA implants immersed with autologous serum showed better hyaline cartilage repair tissue formation compared to microfracture treatment alone in the ovine joint cartilage defect model [17, 18]. Recently, a variety of pilot studies and case reports reported that the PGA-HA implant (chondrotissue®) used in combination with microfracture and autologous serum, PRP or bone marrow aspirates for cartilage repair in the knee or in the talus is safe, improves the patients’ situation, and leads to defect filling with hyaline-like to hyaline cartilage repair tissue [19-23]. In a case series with 52 patients we have shown that implantation of the PGA-HA implants immersed with autologous PRP after subchondral drilling results in significant improvement of the patients’ situation compared to baseline as assessed by the Knee Injury and Osteoarthritis Outcome Score (KOOS) and in the formation of hyaline-like cartilage repair tissue as assessed by histological analysis of repair tissue biopsies, at a one and two year follow-up [24, 25].
Aims of the present study were to follow–up the patient cohort treated with the PGA-HA implants after five years, to assess clinical mid-term efficacy of the procedure by analysis of the patients’ situation using the validated KOOS and to stratify the patients’ outcome with respect to ‘risks’ and patients’ characteristics known to have an impact on cartilage repair like degenerative status of the knee, gender, age, body mass index as well as size and location of the defect.
MATERIALS AND METHODOLOGY
Patients
From August 2007 to January 2009, fifty-two patients with full thickness chondral defects of the knee joint were treated arthroscopically with a cell-free polyglycolic acid-hyaluronan (PGA-HA) implant (chondrotissue®, BioTissue AG, Zurich, Switzerland) immersed with autologous platelet-rich plasma (PRP). In previous reports, patients’ characteristics, inclusion and exclusion criteria as well as the surgical procedure and the clinical outcome at one and two year follow-up have been described in detail [24, 25]. All patients gave consent and were recalled for the 5-year follow-up (range, 58–63 months after surgery). All data were obtained from medical records. As outlined in our previous reports, pre-operative radiographs were evaluated using the Kellgren-Lawrence (KL) scoring system. A Kellgren-Lawrence score of (2 defines osteoarthritis [26] and was found in 26 patients.
Characteristics of patients (32 females, 20 males) with cartilage defects are given in Table 1. The average age of patients at time of surgery was 44 (range 31-65 years). Thirty one patients were 45 years or younger, while 21 patients were older than 45 years. The mean body mass index (BMI) was 24 kg/m2 (range, 19-31 kg/m2). According to the BMI classification of the World Health Organization (WHO), 35 patients were within the normal range of BMI (BMI of<2 5 kg/m2), while 17 patients showed overweight (BMI of ≥25 kg/m2). The mean defect size was 3.0 cm2 (range, 1.5–5.0 cm2). Thirty four patients showed defects of ≤3.0 cm2, and the defects of 18 patients were larger than 3.0 cm2. All defects were classified as ICRS class III (n=16) or IV (n=36) defects [27] and were located on the femoral condyle (n=12) or tibial condyle (n=40). There were no concomitant and no previous surgeries.
Patients' characteristics.
| Characteristic | Patients’ Data |
|---|---|
| Total (n) | 52 |
| Gender | female (n=32), male (n=20) |
| Age (years) | mean 44 (range 31-65),≤ 45 (n=31); > 45 (n=21) |
| Body mass index (BMI) | mean 24 (range 19-31),< 25 (n=35), ≥ 25 (n=17) |
| Height (cm) | mean 167 (range 154-190) |
| Weight (kg) | mean 68 (range 50-108) |
| Kellgren-Lawrence (KL) grading | KL 0 (n=5),KL 1 (n=21),KL 2 (n=15), KL 3 (n=11), |
| Defect size (cm2) | mean 3.0 (range 1.5-5.0),≤ 3.0 (n=34), >3.0 (n=18) |
| ICRS classification | III (n=16),IV (n=36) |
| Location | medial femoral condyle (n=12), medial tibial condyle (n=31), lateral tibial condyle (n=9) |
| Concomitant surgeries | none |
| Previous surgical procedures | none |
Surgical Procedure
The surgical procedure has been described previously [24, 25]. In brief, subchondral drilling as well as implantation of the PGA-HA implants were performed arthroscopically. Damaged and degenerated cartilage was removed using a sharp spoon and a shaver. Drilling (~2 cm depth) into the subchondral bone was performed using a K-wire with a thickness of 1.8 mm. The PGA-HA scaffolds were allowed to incubate in 3 mL autologous PRP (not conditioned PRP; mean concentration, 832.1 x 10³ platelets/µL; 6.1 x 10³ leukocytes/µL) for 5 to 10 minutes. The implants were cut to fit the size of the defect and fixed in femoral defects with Smart Nails® (ConMed Linvatec Italy, Milano, Italy) or in tibial defects with a ‘fibrin glue’-like adhesive made of autologous PRP gelled by calcium gluconate and thrombin additives. The rehabilitation regime has been reported previously [24].
Evaluation of Clinical Results
For evaluation of the patients’ clinical situation, the Knee injury and Osteoarthritis Outcome Score (KOOS, www.koos.nu) [28] was applied pre-operatively and at 5-year follow-up. The pre-operative data as well as the 1-year and 2-year follow-up data have been used for comparison and were reported previously [24, 25]. The KOOS is a patient-administered score and is divided into the sub-categories pain, symptoms (spt), activities of daily living (ADL), sports and recreation function (sport&recr), and knee-related quality of life (QoL). Each sub-category was calculated as the sum of all included items. A score of a maximum of 100 represents no knee problems, while a score of 0 represents severe knee problems. The overall KOOS represents the mean KOOS value calculated from all subcategories. The minimal detectable changes in patients with knee injuries/knee OA are [29]: pain (6.0-6.1/13.4), symptoms (5.0-8.5/15.5), ADL (7.0-8.0/15.4), sport&recr (5.8-12.0), and QoL (7.0-7.2/21.1). The test-retest liability or interclass correlation of KOOS in patients with knee injuries/knee OA is [29]: pain (0.85-0.93/0.80-0.97), symptoms (0.83-0.95/0.74-0.94), ADL (0.75-0.91/0.84-0.94), sport&recr (0.61-0.89/0.65-0.92), and QoL (0.83-0.95/0.60-0.91). The minimal, clinically important change (ΔKOOS) is suggested to be 8-10. To assess cartilage repair and repair tissue quality, 21 patients (14 females, 7 males; defect size 1.5-4.0 cm2; 15 tibial defects, 6 femoral defects; KL score 0-1, n=11; KL score 2-3, n=10) gave consent to additional magnetic resonance imaging (MRI). MRI was performed four years after the surgery with a 1.0-T high speed magnetic resonance imager (General Electric, USA) using multi-linear coil and multi-slice T1-weighted and T2-weighted imaging with fat suppression. Magnetic resonance observation of cartilage repair tissue (MOCART) scoring [30] was performed by a radiologist.
Statistical Analysis
Normal distribution of data was tested using the Kolmogorov-Smirnov method. For comparison of pre- and post-operative KOOS values, the paired t-test and the Wilcoxon signed rank test were performed, depending on the normal distribution of data. For subgroup analysis (Kellgren-Lawrence grading, defect size, defect localization, gender, age and BMI) of the 5-year follow-up KOOS data, the t-test and the Mann-Whitney rank sum test were applied, depending on normal distribution of data. Correlation analysis was done according to the Pearson Product Moment Correlation procedure. The correlation coefficient (r) and the coefficient of determination were calculated (r2). All tests were performed using the statistical software SigmaStat 3.5 (Statcon, Witzenhausen, Germany). Values are given as mean and standard deviation. A ΔKOOS of >10 is considered clinically meaningful and differences/correlations with a p-value of <0.05 are considered significant.
RESULTS
Clinical Outcome
At 5-year follow-up, there were no clinical signs of persistent knee joint infection or inflammation, allergic reactions, foreign body reactions, knee joint effusion or swelling. Temporary blocking was not reported. There were no signs for ablation of the implant or loosening of the repair tissue. Six out of 52 treated patients developed injuries/diseases that were neither related to the knee surgery nor to the implantation of the PGA-HA implant immersed with autologous PRP. One patient was treated with antidepressants at the time of the 5-year follow-up visit. Eight patients reported pain in the treated knee 2-5 years after the procedure. The patients received oral non-steroidal anti-inflammatory drugs (NSAID) and were pain-free when the self-administered patient reported outcomes were recorded. One patient sprained the operated knee during a soccer game approximately 3 years after the surgery. The patient was not subjected to surgery and recovered fully after one month.
Clinical Evaluation of Surgical Results Five Years After Implantation of the PGA-HA Implant Immersed with PRP
Clinical scoring and analysis of KOOS values showed a clinically meaningful (ΔKOOS > 10) and significant (p< 0.001) improvement in all KOOS sub-categories at 1-year, 2-year and 5-year follow-up, compared to the pre-operative situation (Fig. 1). Pain improved from a mean of 54.1 pre-operatively to a mean of 93.5 at 5-year follow-up, symptoms from 56.3 to 91.3, activities of daily living from 68.1 to 89.5, sport&recreation from 35.5 to 74.5, and the subcategory quality of live improved from 37.2 to 76.4. The overall KOOS showed a mean of 50.3 at baseline and increased to a mean of 85.0 at 5 years follow-up, leading to an average ΔKOOS of 34.7. Differences in KOOS values between the 1-year and 2-year follow-up as well as between 1-year or 2-year and the 5-year follow-up values were less than 10 KOOS points and have been considered to be clinically not relevant.
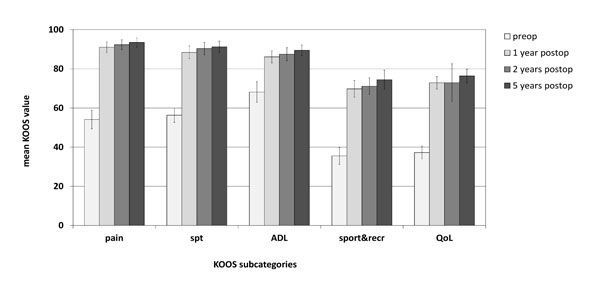
Clinical outcome after five years as assessed by the Knee injury and Osteoarthritis Outcome Score (KOOS). The KOOS profiles prior to and 1 year, 2 years and 5 years after implantation of the PGA-HA implant are presented (n=52). The columns show the mean and error bars define standard deviation (SD). spt, symptoms; ADL, activities of daily living; sport&recr, sports and recreation; QoL, quality of life. Pre-operative KOOS raw data, the 1-year follow-up and the 2-year follow-up data were taken from previous reports [24, 25].
Stratifying the patients’ KOOS data recorded at 5-year follow-up according to the degenerative nature of the treated knee (Kellgren-Lawrence grading), defect size and defect localization as well as gender, age and obesity (Fig. 2) showed that there was no significant (p>0.05) difference in all KOOS subcategories in the group of patients with a Kellgren-Lawrence (KL) grading of 0 or 1 compared to patients with degenerative changes in the knee corresponding to a KL grading of 2 or 3. There were no differences in KOOS related to the size of the defect, gender or BMI. The overall KOOS after subgroup analysis is given in Table 2. Regarding the defect location, there was a significant (p=0.047), but not clinically meaningful difference in the KOOS subcategory pain showing scores with a mean of 92.3 in the group with femoral defects and scores with a mean of 93.9 in the group with tibial defects. Regarding the age of patients, there was a significant (p=0.009) difference in the KOOS subcategory sports&recreation with scores showing a mean of 76.1 in the patient group younger than or 45 years of age and scores with a mean of 72.0 in patients older than 45 years at the time of the surgery.
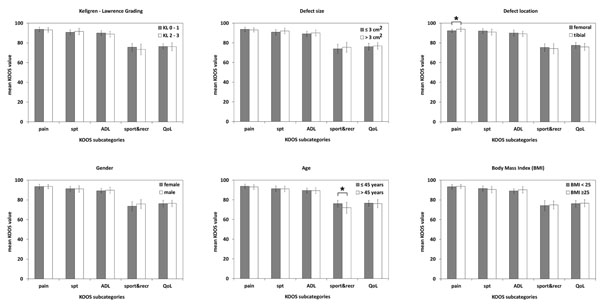
Subgroup analysis of the clinical outcome after five years as assessed by KOOS. Patients’ KOOS data were stratified according to the degenerative status of the operated knee (Kellgren-Lawrence grading 0-1 versus Kellgren-Lawrence grading of 2-3), defect size (≤ 3 cm2 versus > 3 cm2), defect localization (femoral versus tibial localization), gender (females versus males), age (≤ 45 years versus > 45 years), and BMI (< 25 versus ≥ 25). The columns represent the mean and error bars define SD. Asterisks indicate significant (p < 0.05) differences.
Overall KOOS in patients’ characteristics related subgroups.
| Item | Gender (Female/Male) | Age (≤45/>45 Years) | BMI (<25/≥25 kg/m2) | KL Grading (KL 0-1/KL 2-3) | Defect Size (≤3/>3 cm2) | Defect Location (Femoral/Tibial) |
|---|---|---|---|---|---|---|
| Overall KOOS | (84.8/85.4) | (85.5/84.4) | (84.9/85.3) | (85.3/84.7) | (84.7/85.5) | (85.5/84.9) |
Correlation analysis (Table 3) showed that there are significant (p<0.05) correlations between patients’ characteristics such as age, BMI, degeneration status of the knee or defect location and the clinical outcome in some subcategories (activities of daily living, sports&recreation and quality of life). Correlation coefficients and coefficients of determination indicate that the correlations are weak. The correlations between patients’ characteristics and the clinical outcome are considered to be not meaningful.
Correlation analysis.
| KOOS Subcategories | Gender (cc/p-Value), cd | Age (cc/p-value), cd | BMI (cc/p-Value), cd | KL Grading (cc/p-Value), cd | Defect Size (cc/p-Value), cd | Defect Location (cc/p-Value), cd |
|---|---|---|---|---|---|---|
| pain | (-0.02/0.90), 0.0004 | (0.05/0.73), 0.0025 | (0.15/0.30), 0.0225 | (-0.07/0.61), 0.0049 | (-0.12/0.41), 0.0144 | (-0.28/<0.05), 0.0784 |
| spt | (0.03/0.85), 0.0009 | (0.04/0.80), 0.0016 | (-0.09/0.52), 0.0081 | (0.16/0.27), 0.0256 | (0.14/0.32), 0.0196 | (0.16/0.27), 0.0256 |
| ADL | (-0.11/0.46), 0.0121 | (-0.08/0.57), 0.0064 | (0.32/0.02), 0.1024 | (-0.15/0.28), 0.0225 | (0.14/0.33), 0.0196 | (0.12/0.40), 0.0144 |
| sport&recr | (-0.23/0.10), 0.0529 | (-0.51/<0.01), 0.2601 | (0.18/0.20),0.0918 | (-0.34/0.02),0.1156 | (0.06/0.65),0.0036 | (0.10/0.50), 0.0100 |
| QoL | (-0.05/0.74),0.0025 | (-0.27/<0.05),0.0729 | (-0.03/0.85),0.0009 | (-0.10/0.50)0.0100 | (0.14/0.34),0.0196 | (0.20/0.17),0.0400 |
cc = correlation coefficient.
cd = coefficient of determination.
Twenty-one out of 52 patients gave consent to additional magnetic resonance imaging (MRI) four years after implantation of the PGA-HA implant. At four-year follow-up, MRI showed good defect and volume filling with a repair tissue of iso- to hyperintense cartilage signal compared to the adjacent cartilage (Fig. 3A). Some bone signal is evident in the same patient at 2-year follow-up and may be due to pin fixation and/or the drilling procedure (Fig. 3B). The MOCART score was applied by a radiologist for morphological evaluation of the repair tissue (Table 4). At 4-year follow-up, the repair tissue of 20 out of 21 patients showed excellent MOCART scores. One patient developed a repair tissue that showed incomplete defect filling and surface damage.

Representative magnetic resonance imaging after implantation of the PGA-HA implant immersed with PRP. The cartilage defect showed good defect filling with repair tissue of iso- to hyperintense signal compared to the adjacent cartilage at 4-year follow-up (A) and some bone signal in the same patient at 2-year follow-up (B).
Evaluation of cartilage repair tissue (n = 21) at 4-year follow-up by using the magnetic resonance observation of cartilage repair tissue score (MOCART).
| Variables | Points for Scoring | Number of Patients |
|---|---|---|
| Degree of defect repair and filling of the defect | ||
| Complete | 20 | 20 |
| Hypertrophy | 15 | 0 |
| Incomplete >50% of the adjacent cartilage | 10 | 1 |
| Incomplete >50% of the adjacent cartilage | 5 | 0 |
| Subchondral bone exposed | 0 | 0 |
| Integration of border zone | ||
| Complete | 15 | 21 |
| Incomplete, demarcating border visible | 10 | 0 |
| Incomplete, defect visible <50% of the length | 5 | 0 |
| Incomplete, defect visible >50% of the length | 0 | 0 |
| Surface of the repair tissue | ||
| Surface intact | 10 | 20 |
| Surface damaged <50% of depth | 5 | 0 |
| Surface damaged >50% of depth | 0 | 1 |
| Structure of the repair tissue | ||
| Homogeneous | 5 | 21 |
| Inhomogeneous | 0 | 0 |
| Signal intensity of the repair tissue | ||
| Normal (identical to the adjacent cartilage) | 30 | 21 |
| Nearly normal (slight areas of signal alteration) | 15 | 0 |
| Abnormal (large areas of signal alteration) | 0 | 0 |
| Subchondral lamina | ||
| Intact | 5 | 21 |
| Not intact | 0 | 0 |
| Subchondral bone | ||
| Intact | 5 | 21 |
| Not intact (edema, granulation tissue, cysts, sclerosis) | 0 | 0 |
| Adhesions | ||
| No | 5 | 21 |
| Yes | 0 | 0 |
| Effusion | ||
| No | 5 | 21 |
| Yes | 0 | 0 |
DISCUSSION
In the present study, we have shown the benefit and reliability of the use of the cell-free PGA-HA implant chondrotissue® immersed with autologous PRP after bone marrow stimulation for the treatment of full-thickness cartilage defects of the knee. The validated KOOS showed clinically meaningful and significant improvement 5 years after implantation of the implant, compared to the pre-operative situation. There were no differences in the clinical outcome of patients stratified according to typical ‘risk’ factors like degenerative status of the knee, age, weight and gender as well as defect size and location. Magnetic resonance imaging showed good repair with cartilage repair tissue covering and filling the cartilage defect.
Recently, one-step cartilage repair procedures have been developed for the treatment of chondral defects in combination with bone marrow stimulation techniques or bone marrow concentrate [14, 15, 22, 31]. These procedures utilize subchondral progenitor or stem cells, which are released into the defect by bone marrow stimulation, and different types of resorbable scaffolds that may help to keep the cells and the newly formed tissue in the defect. Blood derivatives like serum or platelet-rich plasma (PRP) as well as bone marrow concentrates are added to enhance cartilage repair tissue formation. The original AMIC (autologous matrix-induced chondrogenesis) technique uses a porcine collagen type I/III membrane and fibrin glue mixed with the patient’s blood to cover the microfractured cartilage defect. Clinical efficacy has been shown in a case series of 27 patients with moderate to complete defect filling and an average of 37 months follow-up [32]. However, in a pilot study, it has been shown that the good clinical outcome found after AMIC combined with PRP may not be reflected by magnetic resonance imaging (MRI) that showed persistent subchondral bone abnormalities, incomplete filling or hypertrophy of the repair tissue and intralesional osteophyte formation [15]. In a recent randomized trial, the BST-CarGel procedure that uses a chitosan-based scaffold mixed with whole blood to cover microfractured defects was compared to microfracturing alone. Although the clinical benefit for the patients was equivalent at 1-year follow-up, the BST-CarGel group showed better defect filling and better repair tissue quality than the microfracture group [16].
Here, we have reported the clinical outcome after using the polyglycolic acid-hyaluronan chondrotissue® implant immersed with autologous PRP and combined with bone marrow stimulation (drilling). The procedure led to a significant and clinically meaningful improvement in 52 patients with focal cartilage defects in non-degenerative and degenerative knees, as assessed by KOOS (overall mean KOOS at baseline 50.3 to 85.0 at 5-years follow-up). The excellent clinical outcome as shown by improvement in overall KOOS by 34.7 points compared to baseline is in line with the results reported by another group that used the implant in combination with serum and obtained a mean KOOS improvement of 35.4 points compared to baseline [19]. This suggests that the achievable clinical outcome after cartilage repair with bone marrow stimulation and the PGA-HA implant is better than after microfracture alone. In a recent meta-analysis, it has been shown that the rough estimate for the mean expected treatment effect achieved by microfracture alone is an increase in 22 overall KOOS points [33]. In a randomized controlled study, ACI was compared to the standard microfracture approach for the treatment of cartilage defects of the knee. At 36 months follow-up, the chondrocyte implantation group showed a better clinical outcome with an overall KOOS improvement of 21.25 points than the microfracture group (15.83 points) [34]. At five years follow-up, the clinical outcome was comparable in both groups, but showed a better outcome in subgroup analysis for chondrocyte implantation in patients with less than 3 years since the onset of symptoms. The overall improvement in KOOS was 21.17 points in the chondrocyte implantation group and 14.07 points in the microfracture group [35], while treatment of patellofemoral lesions with ACI resulted in an overall improvement of 26.2 at 4-years follow-up [36]. These data may suggest that our cell-free approach using a PGA-HA implant and autologous PRP may lead to a better clinical outcome than microfracture and may lead to an at least comparable outcome as achieved by cell-based chondrocyte implantation. Nevertheless, randomized and controlled clinical trials are needed to prove that the current one-step cartilage repair approach is superior to bone marrow stimulation and/or chondrocyte or stem cell implantation in cartilage repair.
Subgroup analysis showed that there is a significant difference in the clinical outcome with regard to the defect location in patients with tibial defects, showing less pain than patients with femoral defects. One hypothetical explanation for the difference in pain may be the different types of fixation used in tibial and femoral defects, with pin fixation and additional drilling in femoral defects. However, taking into account that the difference between patients with tibial and femoral defects in the KOOS subcategory pain is less than two KOOS points, the difference may be significant but clinically not meaningful. There was a significant difference in the KOOS subcategory sports and recreation showing a slightly decreased activity level of patients older than 45 years. The decrease in the subcategory sports and recreation is four KOOS points and it is most likely that the activity level of our older patients simply declined because of aging and not of worse response to the procedure in elderly people. However, it has been shown that clinical results after microfracture are age-dependent showing the best prognosis for patients with femoral defects aged 40 years or younger [11]. In autologous chondrocyte implantation, patients’ gender seems to have an impact on the clinical outcome after cartilage repair and a higher BMI (>30kg/m2) is a negative predictor for microfracture-mediated cartilage repair [8, 34, 37]. In the present study, there were no meaningful differences in the clinical outcome after using the chondrotissue® implant in combination with drilling with regard to patients’ gender, age or BMI that was<30kg/m2 in 50 out of 52 treated patients. This finding is in line with a recent study that showed that these risk factors do not affect the survival of microfracture-induced cartilage repair in degenerative osteoarthritic knees, before substantial symptoms arise or a total knee arthrosplasty is needed to be performed [38].
The key limitations of our prospective study are the lack of a control group or comparator and the lack of structural data like histology or delayed gadolinium enhanced magnet resonance imaging (dGEMRIC) [39] that may underline the excellent clinical results by proving the formation of hyaline-like to hyaline cartilage repair tissue. However, the results obtained after 5 years confirmed the good results including MRI and histology found after one and two years [24, 25] and may point toward a good future outcome. In particular, stratification of patients with regard to ‘risk’ factors like the degenerative status of the treated knee showed that the approach leads to good results in traumatic defects and in focal cartilage defects in a degenerative environment (Kellgren-Lawrence grading 2 and 3). Currently, severely degenerated or osteoarthritic defects are not indicated for regenerative cartilage repair techniques. However, ACI procedures have been shown to improve the symptoms in patients with early osteoarthritis and may postpone the need for prosthetic replacement [40-42] and even microfracture has been shown to have the potential to provide pain relief, increase the joint space and form a collagen type II-rich cartilage matrix in older patients with radiological confirmed degenerative changes [43]. Randomized, controlled clinical trials including cohorts with well-defined osteoarthritic defects are still missing to prove that such cartilage repair approaches may improve the patients’ situation in advanced staged osteoarthritis.
CONCLUSION
Our clinical data based on the validated KOOS suggest that the implantation of the polyglycolic acid-hyaluronan implant immersed with autologous PRP in focal cartilage defects after drilling leads to clinically meaningful and significant improvement of the patients’ situation. The clinical results found after 5 years confirm the good findings that have been found in the short-term and suggest that the one-step procedure leads to good lasting results with potential for a good future long-term outcome.
CONFLICT OF INTEREST
CK is employee of TransTissue Technologies GmbH and consultant of BioTissue AG. All other authors declare that there is no financial or other conflict of interest.
ACKNOWLEDGEMENTS
The study was supported by the Eurostars Program (German Federal Ministry of Education and Research, BMBF grant FKZ 01QE1314). The authors are very grateful to Chiara Gentili and Ranieri Cancedda (Università di Genova, Italy) for their unconditioned support and fruitful discussions. The authors thank Dario Confalone (S.C. Radiolgia, Ospedale degli Infermi di Biella) for evaluating MRI.


