RESEARCH ARTICLE
Kümmell’s Disease: Clarifying the Mechanisms and Patients’ Inclusion Criteria
Charalampos Matzaroglou 1, Christos S Georgiou 1, Andreas Panagopoulos*, 1, Kostantinos Assimakopoulos 2, Hans J Wilke 3, Bjoern Habermann 4, George Panos 5, Konstantinos Kafchitsas 5
Article Information
Identifiers and Pagination:
Year: 2014Volume: 8
First Page: 288
Last Page: 297
Publisher ID: TOORTHJ-8-288
DOI: 10.2174/1874325001408010288
Article History:
Received Date: 10/1/2014Revision Received Date: 31/7/2014
Acceptance Date: 11/8/2014
Electronic publication date: 15 /9/2014
Collection year: 2014

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.5/) which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The three major causes of vertebral body collapse include infection, malignant neoplasia, and trauma and it may be difficult to distinguish between them, particularly in the presence of severe osteoporosis. In 1891, however, Dr Hermann Kümmell, further added another possibility of vertebral body crush; the delayed posttraumatic collapse. As originally described, this rare clinical entity includes patients, who after a trivial trauma and an asymptomatic clinical course they develop a progressive vertebral body collapse and a painful kyphosis. Although more than a century has passed from its initial description, only few cases have been reported in the literature, whereas the main pathologic eliciting event is still under investigation. As a consequence, great controversy exists regarding the discrete features of the clinical course, its radiographic appearance and the histopathological findings. To explain the time lag between the initial trauma and the occurrence of the vertebral collapse, the hypothesis of ischemic necrosis was advanced. Equation of Kümmell’s disease with vertebral osteonecrosis, however, has wrongly led many authors to report cases of Kümmell’s disease, even in the absence of history of spinal trauma. On the other hand, high coincidence of vertebral osteonecrosis and the pathognomonic radiographic finding of intravertebral vacuum cleft, has further added to the confusion. In this review we present an overview of the literature on Kümmell’s disease, focusing on the different proposed eliciting mechanisms. We also highlight controversial subjects on clinical course, diagnosis and treatment of this entity, in an attempt to further clarify patients’ inclusion criteria.
INTRODUCTION
Delayed posttraumatic vertebral body collapse, namely Kümmell’s disease (KD), is a rarely reported and poorly documented phenomenon. This eponymous diagnosis represents a posttraumatic vertebral fracture, which is initially asymptomatic, having also unremarkable radiography that gradually become symptomatic and result into vertebral body collapse (VBC) [1]. The lack of adequate imaging and histologic examination, however, between the traumatic event and the eventual vertebral collapse, dissemble the true underlying pathological process. The delayed fashion of the VBC is generally explained by the prevailing hypothesis of ischemic necrosis [2]. This association of KD with osteonecrosis, however, and further connection with specific radiographic findings (intraverte-bral vacuum cleft) has led many authors in wrongly reporting patients with other pathologies, as having KD [3, 4]. On the contrary, KD originally involves patients, without any predisposing disorder for ischemic necrosis, other than trauma [5]. Intravertebral vacuum cleft (IVC), is usually present in KD, but is not exclusive to it [6, 7].
In this report we review the clinical features, diagnostic tools and treatment options of this interesting disorder. We also discuss current concepts on the pathophysiology of delayed posttraumatic VBC. Finally, in an attempt to discern causative factors, we review the radiologic controversy of compression fractures with an intravertebral vacuum cleft.
HISTORICAL PREVIEW
In 1891 a German surgeon, Dr Hermann Kümmell described a series of 5 patients, presenting with a rare clinical scenario: they sustained a minor spinal trauma, then remained essentially asymptomatic for a period of months or, even, years and, eventually, developed a progressive, painful kyphosis at the lower thoracic or upper lumbar regions [1, 5]. Carl Schulz, a student of Kümmell, first assigned his teacher’s name to this condition in 1911. At about the same time with Kümmell, Verneuil (1823-1895), a French surgeon, described a similar condition. In some instances the syndrome has been referred to as “Kümmell-Verneuil disease” [2]. For a period of 35 years, after the original dissertation, KD was struggling for acceptance. With the advent of x-ray, it was recognized, that kyphosis was the result of a delayed VBC. Subsequent authors, however, called into question the existence of the delayed collapse; they thought that the fracture was missed initially due to the poor quality of the radiographic studies. With the papers of Rigler (1931) and Steel (1951) was clearly documented that VBC appears only on delayed films and that initial x-rays are usually normal [8, 5].
The literature on the subject is limited, but recently a renewed interest has been developed. Only 9 cases of KD have been documented, in a total of 14 reports published, since the initial description in 1891 (Table 1).
Cases of Kümmell’s disease reported since 1950.
| Author | Age | Sex | Level | Mechanism | Treatment | Risk Factors |
|---|---|---|---|---|---|---|
| Steel, 1951 [5] | 23y | M | T10 | Spine hyperflexion | Bracing | None |
| Steel, 1951 [5] | 62y | M | T8 | Direct injury | Bracing | None |
| Brower et al. 1981 [9] | 71y | M | T12 | Fall from his own height | Unknown | Osteopenia, alcoholism |
| Hermann et al. 1984 [10] | 45y | F | L1 | Fall from height/direct injury | Unknown | Gaucher Type 1 |
| Van Eenenaam, et al. 1993 [11] | 75y | M | T11 | Heavy-weight lifting | Unknown | Steroids, temporal arteritis |
| Young et al. 2002 [2] | 72y | M | L4 | Shoveling snow | Subtotal corpectomy/ autologous grafting | Diabetes |
| Osterhouse, et al. 2002 [12] | 79y | M | L2 | Indirect injury | Conservative | Steroids |
| Kapoor et al. 2004 [13] | 71y | F | L3 | Fall from a height | Posterior stabilization | None |
| Maheshwari et al. 2004 [14] | 62y | F | L2, L3 | Fall in the bathroom | Unknown | Osteopenia, diabetes |
| Swartz, et al. 2008 [3] | 60y | M | T9, T10 | Minor fall | T9, T10 corpectomies/T8–11 anterior and posterior fusion | None |
| Ma et al. 2009 [15] | 75y | F | T12 | Trivial fall | T12 vertebroplasty | Diabetes |
| I. van der Schaaf, et al. 2009 [16] | 87y | F | L1 | Minor trauma | L1 vertebroplasty | None |
| Matzaroglou et al. 2010 [17] | 31y | M | L1 | Stress fracture | L1 kyphoplasty | None |
| Fabbriciani et al. 2010 [18] | 81y | F | L1 | Spinal trauma | Teriparatide | Osteoporosis |
CLINICAL FEATURES
Steel in 1951 divided the clinical course of KD into five stages [5]. The first stage (initial injury), can be varied in severity and mechanism, while lateral roentgenograms are necessarily negative. The second stage (post-traumatic period) follows with minor symptoms and no limitation in activity. The third stage (latent interval) of relative well-being precedes the onset of progressive disability and usually lasts weeks or months, whereon the patient is not incapacitated. In the different clinical reports of KD, this period varies between 4 weeks and 1 year [2, 3, 5, 9-18]. However, in three cases no asymptomatic interval was observed [10, 14, 18]. In the fourth stage (the recrudescent stage), the patient complains for persistent, localized pain, which progressively tends to become more peripheral with root pain. In the last stage (terminal stage), a permanent kyphosis is formed with or without progressive pressure on roots or spinal cord. The complication of greatest detriment is neurologic compromise, although this finding is rare. However, neurologic findings are more commonly a result of vertebral compression after osteonecrosis, rather simple osteoporotic fractures [12]. KD occurs typically in middle-aged and elderly patients with a slight male predominance [5], although there are few reports in younger individuals [5, 17]. Multiple vertebrae may be affected [3, 14] but a single vertebral involvement is the most common scenario.
Although more than a century has passed since original description of KD, only few cases that fulfill Kümmell’s criteria have been described in the literature (Table 1). They, necessarily, involve patients presented with a delayed VBC and vertebral osteonecrosis after a minor spine injury. Trauma, however, can vary in severity and can be of different nature. In a healthy individual, a minor spinal trauma it may not be noticed or documented at all. The patient may be misdiagnosed with idiopathic avascular necrosis of the vertebral body. This entity has been separately reported [19]. Originally Kümmell, in his series, has described a hypeflexion pattern of spinal injury, after a fall [1]. Most of the cases reported, correspond to this pattern [3, 5, 9, 12-15] or implicate a direct force [5, 10] applied to the spinal column. Van Eenenaam and el-Khoury, on the other hand, reported a case with symptomatic onset of KD due to heavy object lifting [11]. This patient was under steroidal therapy for temporal arteritis, both known risk factors for avascular necrosis (Table 2). Young et al. described another patient, with diabetes mellitus and with symptoms triggered, after shoveling snow [2]. These two reports attribute VBC not to direct or indirect trauma, but to hyperfexion loading of the spine. In another report [17] repetitive weight-lifting and, thus, hyperflexion spinal strain was the causative pattern for KD. Interestingly, this particular patient had no predisposing factors for avascular necrosis. These factors in general can enhance trauma’s effect in vascular supply. However, with varied mechanisms, they can independently trigger avascular osteonecrosis.
Risk factors for avascular vertebral osteonecrosis.
| Osteoporosis | Structural failure/ increased intraosseous pressure/vascular compromise |
|---|---|
| Steroid therapy | Fatty tissue accumulation/crushing of the intramedullary vessels/ microscopic fat emboli in the end-arteries/secondary osteoporosis |
| Hemoglobinopathies | Vascular occlusion/ischemia |
| Vasculitides | Vascular occlusion/ischemia |
| Diabetes | Unknown |
| Alcoholism | Microscopic fat emboli in the end-arteries |
| Cushing’s disorder | Microscopic fat emboli in the end-arteries |
| Malignancy | Unknown |
| Infection | Unknown |
| Post-radiation changes | Direct cytotoxic effect with damage to vascularity |
| Pancreatitis | Pancreatic lipase in systemic circulation/Fat necrosis of bone marrow/ vascular compression/ microscopic fat emboli |
| Cirrhosis | Unknown |
| Sarcoidosis | Unknown |
| Gaucher type 1 | Accumulation of glycosyl ceramide in Gaucher cells/ marrow infiltration/vascular compromise |
RISK FACTORS FOR AVASCULAR OSTEONECROSIS
Avascular necrosis of the vertebral body is usually associated with a known predisposing factor and these cases, without history of spinal trauma, should not be referred as KD. Systemic steroid therapy is considered a consequential predisposing factor. Chronic administration of corticosteroids stimulates hyperinsulinemia, which increases intramedullary fat deposition, resulting in crushing of the intramedullary vessels and vascular disruption of medullary arterioles. Additionally, chronic microfractures of secondary osteopenia and the effect of fatty microemboli are other potential eliciting mechanisms of avascular osteonecrosis and subsequent VBC [19]. Idiopathic osteoporosis, on the other hand, can lead to multiple vertebral compression fractures due to bone structural weakness, but can be complicated also with ischemic osteonecrosis [4]. Other predisposing factors include malignancy, infection, radiotherapy, atherosclerosis, diabetes, cirrhosis, vasculitis and pancreatitis [19, 20]. Hemoglobinopathies, such as sickle cell disease, can result in vascular occlusion and vertebral body ischemia [14]. Cushing’s disorder and alcoholism are well-known risk factors for avascular necrosis, probably because of the presence of microscopic fat emboli in the end-arteries [14]. Finally, Ito et al. reported a case of vertebral body osteonecrosis in a patient with sarcoidosis [21]. The typical noncaseating granuloma in the biopsy specimen of the collapsed vertebra wasn’t observed.
HISTOPATHOLOGY AND PATHOPHYSIOLOGY
Few authors have been reported upon pathologic examination of vertebrae, which had undergone delayed posttraumatic VBC. Schmorl, in 1926, was the first to present autopsy material supporting Kümmell’s hypothesis. The spongiosa of the examined vertebral body was destroyed, resulting in VBC, but no underlying pathological process was found [22]. Cardis et al. two years later described the histopathologic appearance of KD, after pathologic examination of a collapsed L2 vertebral body. The vertebral body was wedge shaped and showed marked atrophy of the bony framework and multiple hemorrhages in the spongiosa on microscopic examination [23]. Kux described “multiple microscopic fractures”, which resulted in the delayed VBC [24].
According to Sweet and Madewell [25], the area of avascular necrosis has four concentric zones: a central zone of cell death, a zone of ischemic injury, a zone of active hyperemia, and a zone of normal tissue peripherally. There is greater activity in the ischemic and hyperemic zones in the early phases, and these zones are subsequently replaced by new bone or fibrous tissue in the later phases. Thus, in the early stages of avascular necrosis, inflammatory cells and fluid are the major findings, while new bone and fibrous tissue predominate in the later stages [26]. This was confirmed by the only clinical case in the literature reported positive biopsies [3]; initial evidence of osteonecrosis was followed by later findings of apparent bony remodeling and medullary fibrosis. Since most patients are middle aged or elderly, degenerative changes and osteoporosis are likely to be universal findings [2]. Interestingly, inflammatory changes and paravertebral fluid collections [3] or soft tissue prominence [11] have, also, been described. Histopathologic signs of osteonecrosis were present in 5 cases sampled for biopsy in the contemporary literature [2, 3, 13, 14, 17]. In three of them signs of new bone formation and marrow fibrosis consistent with bone repair coexisted [2, 3, 14].
As far as the pathogenesis of KD is concerned, there is still no unanimous consensus. Originally Kümmell considered the delayed VBC, a ‘rarefying osteitis’ of inflammatory origin, following a nutritional disorder [1]. He later amended his views; he suggested that the damage to the bone was not the result of inflammation, but always inflicted by the original injury, though not always demonstrable [27]. Steel [5] ascribed KD to multiple, minute trauma of osseus and ligamentous structures, which result in fine cracks and microhemorrhages; these minute ruptures in the spongiosa lead to osteonecrosis. The VBC could be the result of an early strain applied to a deficient material [3,5]. Benedek and Nicholas [28] suggested that the initial trauma cause fine trabecular fractures, unapparent on radiographic imaging; healing will normally occur unless some form of interference is present which can be intrinsic (insufficient vertebral blood supply or herniated Schmorl's nodes) or extrinsic (normal weight-bearing on a weakened bone). Due to this impaired healing process, a vicious circle is developing, leading eventually to the VBC. Another hypothesis, based on spinal angiographies, attributes these structural changes to vascular insufficiency, since the anterior third of the vertebral body is a vascular border zone (“watershed”). The dorsum of the vertebral body receives collateral blood flow while the ventral aspect does not; thus is amenable for ischemic necrosis [3, 29]. Schmorl and Junghanns proposed the concept of osteonecrosis after minute ruptures and hemorrhage into the spongiosa [30].
Currently, avascular osteonecrosis is the prevailing hypothesis for the interpretation of the delayed posttraumatic VBC [2]. The hypothesis of an ischemic necrosis is generally advanced to explain the time lag between the traumatic event and the eventual VBC. This hypothesis was supported by the following arguments: i) findings of bone necrosis was found in the cases sampled for biopsy [2, 3, 13, 14, 17] ii) a history of steroid treatment or alcohol abuse, which are well known risk factors of avascular necrosis of the femoral head, was found in some patients with KD [9-12] iii) coexistence of avascular necrosis of the femoral head and vertebral osteonecrosis was, also, found in some patients [31, 32] and iv) some radiographic analogies between the crescent sign of avascular necrosis of the femoral head and the IVC has been pointed out [33]. Injury seems to trigger vascular supply disruption [5]. However, all these arguments are a subject for discussion. The association of avascular necrosis of the femoral head and ischemic vertebral collapse is not strongly enough supported, as only three cases have been reported in the literature [31-33]. Furthermore, contrary to vertebral osteonecrosis, spontaneous intraosseous gas formation is not observed in cases of avascular necrosis of the femoral head [33]. Pathological findings are also questionable; histopathologic findings of vertebral osteonecrosis might simply be the consequence of a severe vertebral crush fracture [33]. It is not clear, if ischemic osteonecrosis or the vertebral collapse is the first step in the sequence [29, 34]. We found no imaging or histological investigations focusing on the time between the traumatic event and eventual VBC, that could confirmed the ischemic theory. Avascular necrosis as the main pathologic eliciting event still remains hypothetical [33].
DIAGNOSIS
Kümmell’s disease remains a diagnosis of exclusion. Once VBC has been established, a thorough history and general medical evaluation must be obtained [3]. Because VBC can be seen in a variety of other conditions including neoplasm, infection, osteoporosis and predisposing factors to osteonecrosis, laboratory testing should include: complete blood count, complete metabolic panel and especially bone (alkaline phosphatase, serum Ca and P) and liver tests (transaminases, total and direct bilirubin, albumin), erythrocyte sedimentation rate, level of C-reactive protein, tumor markers (CEA, aFP, βhCG, PSA, CA 19-9) and serum electrophoresis. To exclude spinal tuberculosis, a chest X-ray and a skin test are necessary. MRI studies of the spinal column with and without contrast enhancement should always be performed. The MR imaging appearance of avascular necrosis differs from the typical findings associated with malignant neoplasm or infection of the vertebral body [25]. Malignant neoplasms have, also, decreased signal intensity on T1-weighted images and increased signal intensity on T2-weighted images. However the high signal intensity on T2-weighted images is more diffuse, as paravertebral soft tissue involvement may occur [26].
There is no pathognomonic radiographic finding for the diagnosis of KD. The best testing is serial imaging, depicting an initially intact vertebral body after trauma, and then the VBC, as the patient becomes symptomatic. Comparison with old films may help establish whether a compression fracture is acute or chronic, but in the absence of relevant films, a bone scan or MRI can help. Kaufmann et al. reported that fracture duration of up to 1 year was associated with a good response to vertebroplasty [35] and this appears to be verified by other studies [36]. For fractures of uncertain age, an additional indicator of acuteness is marrow edema on MR imaging or increased vertebral-body uptake on bone scanning [37]. In the study by Masala et al. [38], MRI was equivalent to bone scan in selecting patients to be treated with vertebroplasty and kyphoplasty in the first 3 to 4 months, while bone scintigraphy was more accurate in the evaluation of older fractures (> 3/4 months). Thus, bone scan with SPECT or SPECT/CT imaging is helpful for determining activity level in a fracture of unknown age as well as identifying posterior element fractures, additional vertebral body fractures, and even rib fractures [39]. In our experience, even chronic fractures manifesting bone marrow edema or focal radionuclide activity on bone scan may respond to treatment.
On the other hand, although nonspecific, bone scan is considered to be one of the more sensitive imaging tools for the diagnosis of early ischemic necrosis. There are published reports of an observed increased uptake of the radiolabelled osteophilic tracer at the vertebral site being present before the collapse occurred [40] and bone scan has been shown to demonstrate abnormalities during the early phase of KD, when plain x-rays are normal [11]. However, bone scan shows absent or minimal uptake if the lesion is chronic, as the normal osteoblastic response within the osseous reconstruction phase would have been indicative of a recent lesion [17, 26, 41].
Regarding biopsy specimens in cases of a VBC, they are performed only when a malignancy is suspected or as part of sampling and diagnosis confirmation, when a vertebroplasty or kyphoplasty is scheduled. The diagnosis of KD usually does not require bone biopsies.
TREATMENT
Treatment is based on patient’s symptoms and laboratory workout. Because of the rarity of the condition and the paucity of literature, specific treatment protocols are limited. Early reports focused on conservative treatment, while more recent favor surgical intervention. The advantages of surgery include earlier patient ambulation and correction of the kyphotic deformity [15]. Factors that should be taken into consideration include the severity of pain, degree of deformity, and neurologic deficits [2, 15]. In the absence of neurological impairment and with the assumption of an intact posterior vertebral wall, conservative pain management with analgesic drugs, bed rest and bracing can be tried out [2]. In selected cases teriparatide, a recombinant form of parathyroid hormone may be considered a valid treatment to fill the osseous gap, relieve pain and resolve the related disability [18]. When nonoperative treatment fails or in cases with great kyphotic deformity, minimally invasive procedures (vertebroplasty or kyphoplasty) are indicated to eliminate the motion at the fracture site, to restore spinal alignment and to relieve pain. For vertebroplasty the patients are positioned in prone position with hyperlordosis. Hyperlordosis induces opening of the cleft and restores the height of the vertebral body [16, 42]. To prevent cement leakage, cavity-grams with injection of contrast medium may be obtained prior to cement insertion [16]. Complete filling of the cleft is advocated to maximize stabilization of the fracture [16, 43]. Some propose leaving patients in the prone, hyperextended position for an additional time of 30 minutes after cement injection to prevent any unnecessary motion along the fracture site [43]. The results after vertebroplasty, for fractures with or without clefts, are quite controversial especially regarding kyphosis correction and cement extrusion [15, 42, 44]. In a study by Krauss et al. [42] who performed vertebroplasty in 44 clefted fractures and 148 vertebrae without cleft, higher reduction of kyphosis angle and lower cement leakage was found in the clefted group. Opening of the IVC with hyperlordosis was considered responsible for better reduction, thus making kyphoplasty unnecessary. The lower rate of cement leakage was attributed to the fact that IVC is an avascular bone area, surrounded by a fibrocartilaginous membrane. However, only 3,1 ml of cement per vertebra was used in this report. Ha et al. [44] presented their results of percutaneous vertebroplasty in 39 patients without IVCs and 12 with IVCs. They found also greater initial correction in the clefted group. However, loss of reduction was greater in the same group postoperatively. In contrast to the previous report, they found more leakage in the presence of a cleft, especially type-C leakage through cortical defects. Pain reduction showed no significant difference between both groups, in both reports. However patients with an IVC exhibited higher pain scores in the second report and, thus, more inconvenience in daily life after the operation than those without a cleft.
For chronic VBC or acute VBC with posterior wall disruption surgical stabilization via fusion should be applied. If there is evidence of neurologic compromise, then definitive decompression with stabilization should be performed. The concern with decompression alone, such as laminectomy, is the progression of the kyphotic deformity, which can lead to further complications, thus making stabilization indispensable [45]. Decompression can be approached either anteriorly [46] or posteriorly [47]. Favorable results have been reported using anterior decompression and fusion with intervertebral tricortical graft [48] or ceramic glass spacers [46]. The advantage of an anterior approach is the technical ease for removing the retropulsed fragment. However, in elderly patients where comorbidities may be significant, violating the thoracic cavity or the retroperitoneal space can have severe consequences; performing a posterior procedure only would, thus, be advantageous [15, 49]. Manual reduction of fracture with transpedicular insertion of vertebral body augments (a titanium spacer with bone-ingrowth porous surface) combined with short segment fixation has also been described for KD with cord compromise [50].
MANAGEMENT OF CHRONIC BACK PAIN
The main symptom of KD is the chronic pain, which continuously increases. Treatment options for patients with chronic back pain are limited. Exercise therapy and non-steroidal antiinflammatories can be beneficial, but it is uncommon for these treatments to lead to a complete resolution of the pain [51]. The biopsychosocial model is gaining acceptance in low back pain; psychological factors, notably distress, depressive mood, anxiety and somatization, are implicated in the development of chronic low back pain. All these factors contribute towards emotional reactions and pain; as a consequence, treatment of one improves the other [52]. In the face of extreme pain, the role of personality factors, affective states and environment become less important, so the psychiatrist should avoid psychological diagnoses and, hence, minimize patients’ complaints in the eyes of others. Patients with psychological problems are more difficult to assess and their complaints are more easily disqualified; hence the consultant must encourage the medical staff to manage their pain as aggressively as that of other patients [52]. The psychiatric diagnosis must be explained, to both patients and the medical staff, its impact on sick behavior must be clarified and a treatment strategy must be decided. The psychiatric team should be able to propose ways of optimizing pain treatment, until surgery is performed. This may include cognitive-behavioral methods, supportive psychotherapy and psychiatric medical treatment. Behavioral interventions increase the sense of control and decrease anxiety and pain, whereas psychotherapy aids in differentiating between pain and suffering and encourages appropriate response to both [53]. Pharmacotherapy with new antidepressant agents is also useful until surgical management is decided [54].
KÜMMELL’S DISEASE AND INTRAVERTEBRAL VACUUM CLEFT
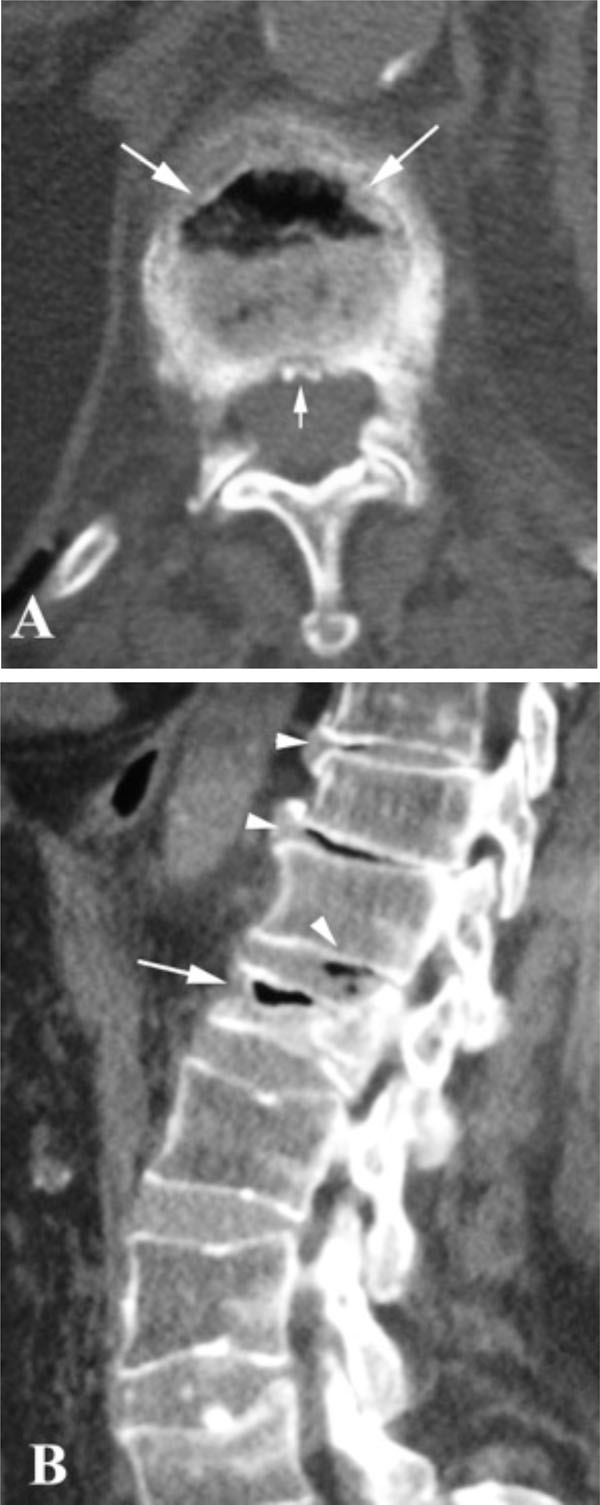
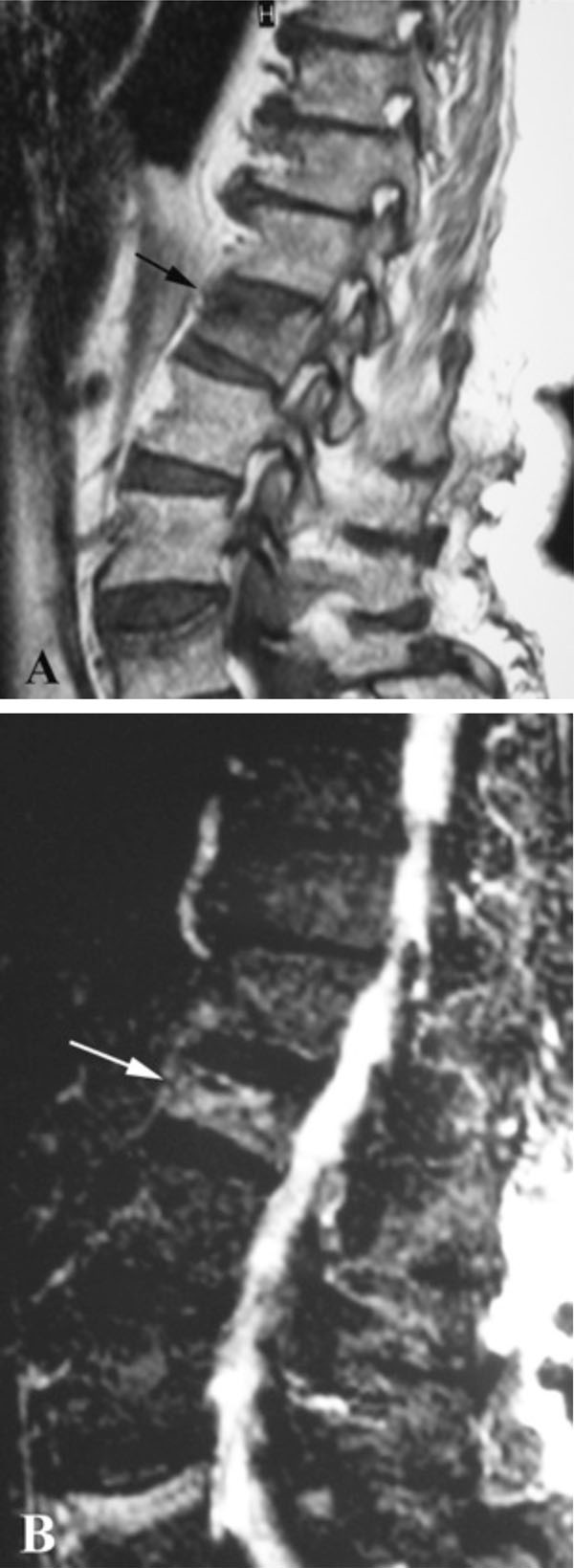
Despite the considerable amount of literature, a definite correlation between IVC with vertebral osteonecrosis and KD has not been established yet. An Intravertebral Vacuum Cleft is characterized by a radiolucent zone within the vertebral body, filled with gas (Figs. 1, 2). The gas is 95% nitrogen, with small amounts of oxygen and carbon dioxide [55]. However, after prolonged supine positioning, it is replaced by fluid, resulting in a high intensity band on T2-weighted sequences (Fig. 3) [56]. Analysis of this serous fluid showed a consistency similar to synovial fluid [57] or plasma [14, 58]. Furthermore, biopsies of the cavity lining demonstrated fibrocartilage within a fibrous stroma, findings that represent a pseudosynovium of a nonunion [57, 59].
In the past, the presence of such a cleft in an adult patient was considered a pathognomonic radiological sign of vertebral osteonecrosis [33]. Horizontal linear IVCs were suggestive of a benign collapse and had never been associated with acute fracture, infection, or malignacy (either primary or metastatic) [27, 33]. In the series of Maldague et al. [33] in 14 patients with non-ischemic causes of vertebral collapse (five with metastatic collapse, five with diskitis, and four with simple traumatic fractures), no IVC was present initially and none could be elicited with extension views.
Acute fractures presumed not to show IVCs, as the space between the bone fragments is initially occupied by the fracture hematoma, followed by callus formation. Further confirmation that IVC is related to avascular necrosis came from the study of Libicher et al. [34]. They carried out a correlated radiological and histological evaluation of 180 cases of vertebral compression fractures. They found ischemic osteonecrosis on biopsy specimens in 11 of the 12 patients with IVC; only one of the 167 patients without osteonecrosis showed IVC. The resulted cleft sign’s sensitivity was calculated at 85%, while specificity at 99%.
Recent studies, however, have shown that these clefts are not so rare and are frequently associated with osteoporotic fractures [6, 60]. An incidence of 10-48% has been reported in patients with osteoporotic compression fractures recruited for vertebroplasty [60-62]. The pathogenetic mechanism is still controversial with three possible theories favored in the literature. According to the first, intravertebral vacuum phenomenon is the result of vertebral osteonecrosis [26, 33]. Ischemic collapse leads to an overall decrease in the volume of vertebral bone, resulting in intraosseous cleft formation; the generated low pressure in these clefts allows accumulation of gas and produces the IVC phenomenon [63]. However, the high incidence of clefts in osteoporotic fractures makes unlikely a causal connection of these clefts with ischemic necrosis [43]. The second theory supports that intravertebral vacuum phenomenon represents nonunion and pseudarthrosis of a vertebral fracture [6, 57, 59, 60]. A third theory, which, however, had limited acceptance, suggested that intravertebral gas could be of intradiskal origin, since a high coincidence (83%) of intervertebral disc vacuum was noted in cases of IVC (Fig. 2B) [31]. Spinal infection, on the other hand, may rarely be accompanied by intradiscal or intraosseous gas so that the latter finding does not entirely exclude the possibility of infection [64]. Intravertebral vacuum has not been indeed reported in the context of acute spinal trauma, in contrast to intradiskal gas [65]. However, if one defines as acute fractures those with symptoms onset less than 3 months [66], IVC is found in such fractures [57, 66, 67]. Furthermore, in a review of spinal radiographs of 2000 patients, Kumpan et al. [7] provide the only description within the literature of an IVC in malignant collapse. They found IVCs in 17 of 2000 patients (<1%). Two of these patients were known to have multiple myeloma and neoplastic involvement of the vertebral bodies, which exhibited the vacuum phenomenon confirmed at autopsy. Moreover, one of these patients had visible IVC only after the application of traction. The other patient had a central, rounded radiolucency. It must be noticed, that these round clefts associated with malignancy were reported during traction of the patients, and it is not clear if the IVC was linear in plain films without traction. The authors consider the IVC a nonspecific finding, which does not exclude the presence of malignancy in the affected bone, as previously advocated [27, 34]. Gagnerie et al. [68] also described a patient with multiple myeloma and a linear IVC in a vertebral body collapse. Histologic evaluation, however, showed evidence of ischemic necrosis only, without signs of myelomatous involvement of the affected vertebra. It must be emphasized that a round intravertebral gas collection is not diagnostic for multiple myeloma; a globular-shaped vacuum cleft have also been found in cases of vertebral osteonecrosis [12], while vertical clefts have been noted in simple osteoporotic fractures [6].
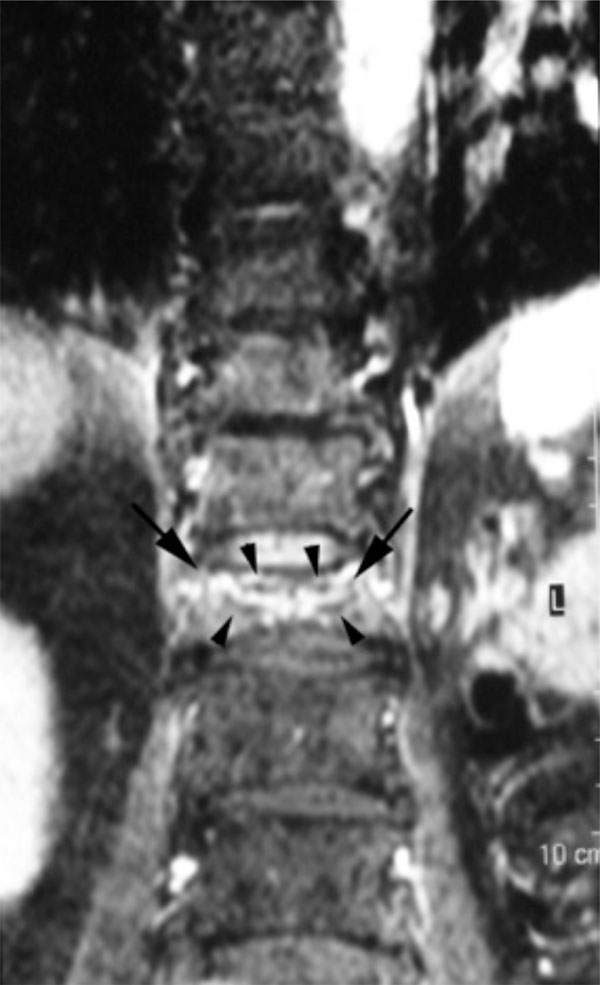
IVCs demonstrate dynamic mobility in different body postures thus indicating instability within the fracture [62, 69]. The dynamic mobility of IVCs is expressed by the changes in vertebral body height that can be seen on standing lateral and cross-table supine lateral radiographs [18, 69]. In some cases, the cleft only appears in extension stress views and disappears in flexion [33, 56]. This motion within the fracture has been correlated with a high probability of severe, persistent pain [6]. Thus, an accurate diagnosis of an IVC requires not only standing lateral, but also extension (cross-table lateral supine) projections, in order to avoid false negative results. Clefts are identifiable with standing lateral radiography, supine cross-table radiography and MRI, at 14%, 64% and 96%, respectively [62]. However, several IVCs remain undetected, until they are filled with opaque cement at the time of vertebroplasty [43]. On the other hand, the signal intensity of the gas noted on plain radiographs might be different from the expected low signal intensity on T1 and T2 MRI sequences. After prolonged supine positioning, the clefts can be filled with fluid, resulting in a high intensity band on T2-weighted sequences (Fig. 3) [56]. This linear, horizontal, increased on T-2 weighted images signal, follows the same pattern with the signal in cases of avacular necrosis of the femoral head [66]. It is probably a result of prolongation of the T2 relaxation time of the fluid and inflammatory exudate during the early phases of avascular necrosis [25, 70]. This sign has been recognized in all reported cases of KD, when MRI study was available [2, 3, 13-16]. However, in patients with later phases of new bone and fibrous tissue formation, a linear area of hyperintensity on T2-weighted images cannot be identified [17, 26]. Additionally, a peripheral zone of hypointensity can be seen surrounding the hyperintensity on T-2 weighted images (Fig. 4) [2, 3, 14, 16]. This finding is termed “double line sign” and corresponds to the IVC [26]. It is thought to represent sclerosis surrounding central granulation tissue and has also been described in cases of avascular necrosis of the femoral head [3, 70].
In conclusion, IVCs represent gross disruption of cortical and cancellous vertebral bone and are time and position dependent, resulting in a variable radiographic appearance. A conflation of pseudarthrotic and osteonecrotic theories could explain their presence [39]. A severe crush fracture of the osteoporotic vertebra could lead to necrosis, if the motion remains. Alternatively, ischemia of the weakened osteoporotic trabeculae could cause a fracture, which would not heal due to the insufficient blood supply and can lead to pseudarthrosis [39]. Clefts can be also observed in a variety of concomitant disorders, benign or malignant. The absence of an IVC, does not exclude avascular necrosis from the differential diagnosis [12, 17]. Therefore, a vertebral cleft is not specific for KD, thus the IVC shouldn’t be referred as Kümmell’s sign [3, 4, 62, 69].
CONCLUSION
KD is the eponymic designation for the delayed posttraumatic VBC. Although it is weakly validated, hypothesis of ischemic posttraumatic vertebral necrosis is the prevailing theory, which tries to explain the delayed fashion of the VBC. Furthermore, the cause, symptoms, and diagnosis of KD are very individual. There is still no consensus about the nature and how severe the initial trauma has to be, how long the asymptomatic period should be and what types of tests should be used to support the diagnosis. However patients, even young individuals, who have refractory symptoms, after seemingly innocuous thoracolumbar spinal trauma or prolonged spinal hyperflexion loading, should have repeated follow-up radiographic evaluations, even though initial studies were deemed normal. This is particularly true in patients who exhibit potential risk factors for avascular osteonecrosis, notably advanced age, osteoporosis and chronic steroid usage. If a VBC is recognized, patients should undergo an extensive work up to exclude other potential underlying conditions, mainly malignancy or infection. For the nonoperative management of chronic back pain of KD, as well as of simple osteoporotic fractures, interdisciplinary approach with biomedical and psychosocial tools is mandatory. When conservative treatment fails, percutaneous vertebroplasty seems to be the current standard surgical therapy. In this case, it may be important to distinguish cases
of KD from typical compression osteoporotic fractures, since the severe destruction of the vertebrae, associated with ischemic collapse, may increase the risk of retropulsion of bone or methylmethacrylate into the spinal canal during vertebroplasty.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.