All published articles of this journal are available on ScienceDirect.
Tranexamic Acid Reduces Blood Loss and Transfusion in Patients Undergoing Total Knee Arthroplasty without Tourniquet: A Prospective Randomized Controlled Trial
Abstract
Introduction :
Blood loss during and after total knee arthroplasty (TKA) can lead to substantial morbidity and the need for blood transfusions. There are several methods to minimize blood loss and to decrease transfusion rates in patients undergoing TKA. Tranexamic acid is an antifibrinolytic agent with known efficacy for achieving these goals. Currently, many surgeons are performing TKA without the use of tourniquet. Consequently, the aim of the study is to evaluate whether tranexamic acid reduces blood loss during and after TKA without the adjunctive use of above-the-knee tourniquet.
Methods :
We performed a prospective randomized controlled trial (1:1 fashion) on the use of tranexamic acid versus placebo in 50 patients undergoing TKA (without tourniquet). The treatment group received two (preoperative and postoperative) 15 mg/kg doses. The primary endpoint was blood transfusion rate. We collected data about demographic and procedural characteristics, hemoglobin and hematocrit values, drain blood loss at 24 hours as well as adverse events.
Results :
There were no transfusions in the treatment group, whereas 32% of the control group required transfusion (p<0.01). The treatment group had higher hematocrit and hemoglobin levels at 24, 48 and 72 hours after surgery (all p<0.01) and lower drain loss at 24hours (363.4±141 vs 626±260ml, p=<0,001). There were no in-hospital or six-month thromboembolic complications.
Discussion :
A double-dose of tranexamic acid was safe and effective, reducing blood loss and preventing the need of blood transfusion in patients undergoing TKA without above-the-need tourniquet.
INTRODUCTION
Bone and soft-tissue bleeding (600-1500 cc) represent the most common cause of postoperative morbidity after total knee arthroplasty (TKA), increasing transfusion requirements up to 50% and prolonging length of hospitalization [1-6]. Moreover, the widespread adoption of antiplatelet and anticoagulant agents to reduce thromboembolic events in patients undergoing TKA has considerably increased bleeding risk. Tranexamic acid is a synthetic derivative of the amino acid lysine that inhibits fibrinolysis by competitively blocking the lysine-binding sites of plasminogen. Numerous studies have demonstrated that the administration of tranexamic acid, either topically or systemically, diminishes bleeding following an array of surgical procedures [7-10], including TKA [3, 11], without predisposing to thromboembolic complications.
The application of an above-the-knee pneumatic tourni-quet during TKA can also reduce intraoperative bleeding [12]; nonetheless, this practice does not reduce overallblood loss [13] and provoke considerable tissue damage and postoperative pain [14], which may also slow rehabilitation. In addition, its use for more than an hour during TKA increases the risk for local arterial and venous thrombosis [15]. Meanwhile, the introduction of modern cementing techniques and the use of tranexamic acid may obviate the need for a tourniquet during TKA [16]. In our institution, we performed this procedure without applying a tourniquet. We are not aware of any prospective, randomized, controlled trial that has evaluated the efficacy of tranexamic acid in reducing blood loss in patients undergoing TKA without the adjunctive use of pneumatic tourniquet. Therefore, we designed a randomized controlled study to evaluate the use of this agent in patients undergoing TKA without tourniquet. The primary outcome measure was transfusion rate, secondary outcome measures were drain output, hemoglobin/hematocrit levels.
METHODS
The present study received institutional review board approval, informed consent was obtained from all patients. We enrolled patients in a consecutive prospective manner on a voluntary basis. The study was conducted between September 2011 and July 2012. Candidates for the study were patients with a diagnosis of osteoarthritis scheduled to have primary, unilateral TKA. All patients had normal preoperative platelet count, normal prothrombin time, normal partial thromboplastin time, normal international normalized ratio. Patients were excluded if they had allergy to tranexamic acid, a prior history of thromboembolic disease, congenital or acquired coagulopathy, renal or liver dysfunction, myocardial infarction within the last 6 months or retinopathy. Patients taking antiplatelet agents were asked to stop them at least 7 days before surgery.
We randomized the patients to the study drug or placebo in a 1:1 fashion. The treatment group received tranexamic acid 15mg/kg (diluted in 100 cc of normal saline) 10-minute intravenous infusion twice, the first dose during induction of anesthesia and a second three hours later. The randomization was performed on the day of surgery from a set of 50 envelopes (25 envelopes for each group). A junior resident picked the envelope on the day of the surgery and conveyed the envelope to the anesthetist.
Mean age was 71.5±9.4 years (range 43-90 years) in the treatment group and 72±6.8 years (range 61-84) in the control group, p=NS. Female gender was prevalent in both groups (60% for treatment and 80% for control group; p=0.NS). Preoperative risk according to the ASA (American Society of Anesthesiologists) was as follows: ASA II in 47 patients and ASA III in 3, without signficant differences between treatment groups.
Procedure
All patients had spinal anesthesia. A dose of 2 g cefazolin was given intravenously shortly before the operation. Surgery was performed without the use of above-the-knee tourniquet. An anterior skin incision from the upper border of the patellar to tibial tubercle was used in all cases. Internal and external capusulotomies were performed for genu varo and valgo, respectively. All patients received a posterior stabilized cemented prosthesis: 19 with Scorpio® (Stryker, Kalamazoo, MI, USA), 20 with PFC® or Tc3-PFC Sigma®(DepuySynthes, Warsaw, IN, US), 5 with NexGen High Flex® (Zimmer Inc., Warsaw, In, USA), 3 with Advanced Medial Pivot® (Whright Medical Technology Inc., Arlington, TN, USA), 1 with Vanguard®(Biomet, Warsaw, IN, USA), 1 with Raven® (Fico, Buenos Aires, Argentina) and 1 with Kinetron III® (Ortosintese, Sao Paulo, Brazil). All patients received cemented prosthesis and the patella was not resurfaced in any case. In each knee, one intraarticular drain (10-gauge) was used and connected to a high-vacuum drain bottle. In all cases, knees were placed in compressive bandages and splint. The patients were asked to perform a mechanical ankle pumping exercise regimen for deep vein thrombosis prophylaxis as soon as possible. The compressive bandages and the splint were removed on the first day after surgery and the patient could start ambulation with a walker. All patients received subcutaneous enoxaparin 40 mg for 30 days starting 12 hours after surgery.
Outcome Measures
The primary endpoint was transfusion rate during hospitalization. Patients were transfused in the event of a hemoglobin level <8 g/dl or <9 g/dl associated with either patients instability or with prior cardiac history. We also recorded data regarding the total drain output at twenty-four hours and the serial changes in hematocrit and hemoglobin (baseline, first, second and third postoperative days) values for the hospital stay as well as adverse events both related to the wound and to any medical complications.
Sample Size
Tranexamic acid possesses a powerful effect on bleeding and may abolish transfusion requirements. Thus, we calculated our sample size with use of the following inputs: 90% power, a critical p value of 0.05, 100% reduction in transfusion rate. This resulted in a necessary sample size of 22 subjects per group, for a total required sample size of 44 subjects. We added a 10% failure rate for improper data collection, giving us a total needed sample size of twenty-five subjects per group, or 50 for the study.
Statistical Analysis
We analyzed group differences in the study baseline and procedural (operative time) characteristics along with endpoint parameters (transfusion requirements, drain loss at 24 hours, serial changes in hemoglobin and hematocrit levels). Data were expressed as mean + standard deviation (SD). A two-tailed unpaired t test (or Mann-Whitney U test for nonparametric data) was used to assess differences in continuous variables. Comparisons between continuous variables were performed with paired t test or analysis of variance. Chi-square or Fisher exact was used between groups. A p<0.05 was considered significant.
RESULTS
Mean operative time was 91.8±10.9 minutes for the treatment and 93.2±9.4 minute for the control groups (p=0.629). Mean hospital length was 4.1±8.3 days (range 3-7 days) and 3.8±9.4 days (range 3-7 days) for the treatment and control groups (p=0,271), respectively.
Postoperatively, hematocrit and hemoglobin levels were significantly higher in the treatment group (Fig. 1A, B and Table 1). At 24, 48 and 72 hours, both hematocrit and hemoglobin absolute drops were lower in the treatment group (Fig. 1C). In addition, blood drained during the first 24 hours was lower in the treatment group (363.4±141 vs 626±260 ml; p<0.001). This 42% absolute reduction in drain loss resulted into zero transfusion requirement for the treatment group and 32% (8/25, 2.32 odds ratio, 95% confidence interval 1.56-3.44) in the controls (p=0.002); with 2.12 (range 1-4) transfused units. Six out eight transfused patients had a postoperative hemoglobin level < 8 g/dl, while 2 patients had a hemoglobin <9.5 g/dl associated with significant comorbidities. None of the patients in the treatment group reached these transfusion thresholds. During hospitalization, we suspected deep vein thrombosis in 12 cases (7 in treatment group and 5 in control group), but sonographic evaluation was negative in all cases. There were no thrombotic, embolic or infectious complications during hospitalization and at six-month follow-up.
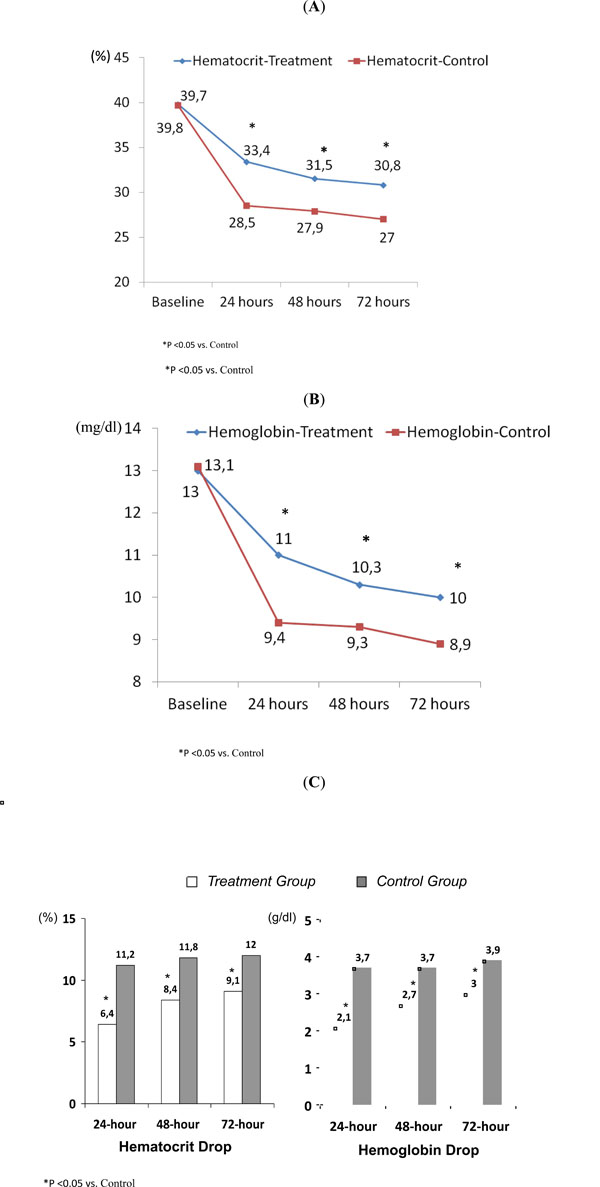
Hematocrit (A) and hemoglobin (B) values at baseline, (C) 24, 48 and 72 hours after surgery.
Serial hematocrit and hemoglobina values in both groups.
| Treatment Group | Control Group | p-Value | |
|---|---|---|---|
| Baseline | |||
| Hematocrit | 39.8±3.4 (33-46) | 39.7±3.45 (34-46) | 0.79 |
| Hemoglobin | 13±1.1 (11.3-15.4) | 13±1.1 (10.4-15.4) | 0.91 |
| 24-hour | |||
| Hematocrit | 33.4±3 (27.1-29.9) | 28.5±3.4 (27.1-29.9) | <0.001 |
| Hemoglobin | 11±1 (10.5-11.4) | 9.4±1.3 (12.6-13.7) | <0.001 |
| 48-hour | |||
| Hematocrit | 31.5±3.3 (30.1-32.8) | 27.9±2.4 (26.8-28.9) | <0.001 |
| Hemoglobin | 10.3±1.2 (9.8-10.8) | 9.3±0.9 (8.9-9.7) | 0.004 |
| 72-hour | |||
| Hematocrit | 30.8±3 (29.5-32) | 27±2.9 (25.6-28.4) | <0.001 |
| Hemoglobin | 10±1.1 (9.6-10.5) | 8.9±1.1 (8.4-9.4) | <0.001 |
Values are shown as mean ± standard deviation (range).
DISCUSSION
TKA is associated with considerable intraoperative and postoperative bleeding and the subsequent need for blood transfusion [1-3]. Several studies have demonstrated that blood transfusion carries an excess risk of infections, arterial and venous thrombosis along with untoward immunologic reactions [17, 18]. Besides, blood transfusion increases overall procedural cost. Several strategies have been used to diminish transfusion requirements such as the use of pneumatic above-the-knee tourniquet, postoperative blood salvage [19], hypotensive anesthesia, use of femoral intramedullary plug [20], cryotherapy [21] or use of Jones bandage [22] along with the administration of topical agents (fibrin-based or thrombin-based).
Tranexamic acid affects the fibrinolytic system by inhibiting the proteolytic action of plasmin, which stabilizes clot formation and diminishes blood loss. Intravenous administration of this agent has dramatically reduced the rate of bleeding during TKA [23]. Several investigators have also demonstrated its efficacy as a topical agent (i.e. dental procedures in patients with hemophilia or on oral anticoagulation [10], orthopedic surgeries [24, 25], or trauma-associated bleeding [26, 27]). Nonetheless, Ahlberg et al. reported that the drug diffuses rapidly through the synovial membrane, which allows for an immediate anti-hemorrhagic effect during TKA [28]. In a recent TKA study [29], five different intravenous drug regimens were tested using 10 mg/kg doses: (1) intraoperative single-dose; (2) double-dose, intraoperative and postoperative (3 hours after the first dose); (3) double-dose, preoperative (20 minutes before tourniquet inflation) and intraoperative; (4) triple-dose regimens, preoperative, intraoperative and postoperative; and (5) placebo. In this seminal study, regimens (3) and (4) showed the best anti-bleeding effect [29]. This study clearly suggests the need for an intravenous preoperative dose of tranexamic acid to obtain the strongest effect [29]. In line with these results, we used double-dose scheduled regimen with a preoperative dose and second dose 3 hours after the procedure, but in contrast with prior studies, we did not apply tourniquet. In this setting, the drug regimen was extremely effective in reducing blood loss and transfusion requirements. To our knowledge, this study is the first to test the clinical safety and efficacy of this agent in patients undergoing TKA without above-the-knee pneumatic tourniquet. The absence of cases with symptomatic deep vein thrombosis is reassuring; however, it is not surprising giving the excellent drug profile shown so far. Although speculative, the avoidance of blood transfusion with this agent may reduce thrombosis risk.
In our study, transfusion requirements were low (0% with tranexamic acid and 32% without) compared to a recent meta-analysis (29% with tranexamic acid and 55% without) [3], despite similar drain loss (319 and 630 ml with and without tranexamic acid, respectively). Discrepancy in transfusion rates between our study and others may be due to differences in clinical threshold for blood transfusion.
In the present study, several limitations deserve mentioning. Because of the open-labeled nature of the study, we cannot exclude specific biases regarding surgical decisions during the procedure or the need for blood transfusion. Nonetheless, we used pre-specified indications for blood transfusion. Sample size does not allow for clinical outcome comparison between groups, however, severe postoperative bleeding after TKA leads to a worst short and long-term outcome. Our findings only apply to this type of TKA surgery, thus, they should not be generalized to other orthopedic procedures.
In the present study, we used ultrasound to identify deep vein thrombosis below the knee. This non-invasive imaging tool though has a low sensitivity for deep vein thrombosis.
By measuring blood loss in the drains, we quantified only a partial amount of total blood loss. In addition, we used a somewhat arbitrary dose of tranexamic acid, different drug regimens may warrant further evaluation against the present regimen. Lastly, we did not perform a cost analysis; nonetheless, the study drug is affordable. Additionally, tranexamic acid should prove cost-effective owing to the dramatic reduction exerted by the drug in terms of postoperative bleeding and the need for transfusion observed.
In conclusion, tranexamic acid reduces postoperative blood loss and the need for blood transfusion in patients undergoing TKA without the adjunctive use of an above-the-knee pneumatic compression tourniquet. We observed no increase in symptomatic thromboembolic phenomena in our patients.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


