All published articles of this journal are available on ScienceDirect.
Treatment Failure Among Infected Periprosthetic Total Hip Arthroplasty Patients
Abstract
Two-stage revision has been shown to be the most successful treatment in eradicating deep infection following total hiparthroplasty. We identified 62 patients treated by a two-stage revision. We defined “successful revision” as negative intraoperative cultures and no further infection-related procedure. We defined “eradication of infection” on the basis of negative cultures and clinical diagnosis at least one year after 2nd stage procedure. After a mean follow up of 2.7 years, eradication of the infection was documented in 91.1%, and a successful two-stage revision in 85.7% of patients. We observed no association between higher pre-reimplantation levels of ESR and C-reactive protein and lower likelihood of successful two-stage revision. We found an association between a history of another previous infected prosthetic joint and a failed 2nd stage procedure. Failure to achieve eradication of infection and successful two-stage revision occurs infrequently. Patients with prior history of a previous prosthetic joint infection are at higher risk of failure.
INTRODUCTION
Total hip arthroplasty (THA) has become the gold standard treatment for patients with end stage arthritis [1]. The benefits of total hip arthroplasty as a treatment for arthritis have been well documented. Multiple studies have demonstrated that following total hip arthroplasty patients experience significant quantitative and qualitative improvement in both their physical function and quality of life [2-4]. Utilization of THA is rising with current projections in the United States predicting an increase of 174% to nearly 600,000 THA procedures annually by 2030 [5-7].
Postoperative infection is one of the most devastating complications encountered after THA. In the initial studies of THA, the lifetime infection rate was found to be 9%-12%, and the procedure was nearly abandoned [8]. However, with improvement in sterile procedure and preoperative antibiotic prophylaxis, the overall lifetime risk of infection has been reported to decrease to less than 1% [9]. However, a report using Medicare data documented a 1.63% rate of infection in THA within the first two years, with most cases presenting in the first 4 weeks postoperatively [9].
Treatment options for infected THA depend on the chronicity of the infection, patient comorbidities and type of organism. Treatment options include antibiotic suppression, debridement and irrigation and exchange of all modular parts, single-stage revision, two-stage revision, and resection arthroplasty (Girdlestone). All of these treatments are accompanied by long term intravascular antibiotic treatment protocols.
Two-stage revision has been shown to yield a high rate of infection eradication and survival of the reimplanted THA and is the standard of care in North America for infected THA [10, 11]. An articulating antibiotic spacer helps deliver local antibiotics, maintains joint mobility, and facilitates reimplantation by reducing soft tissue contracture and scar formation [12]. Studies using a two-stage revision with an articulating spacer have reported success rates ranging from 89%-96% [10, 13, 14].
We report a large series of patients with deep infection following THA managed by a single surgeon with a highly specialized revision THA referral practice. The surgeon used a standardized surgical technique and treatment protocol, including a custom intraoperative fabricated articulating cement spacer constructed from a custom-made mold. Intraoperative fabrication of the cement spacer with a customized mold allows the surgeon the freedom of utilizing different cement types, choosing the added amount and type of antibiotics, and tailoring the mold to the patient’s anatomy. This enables a better femoral canal fit and antibiotic delivery. We report the rate of infection eradication and successful two-stage revision, as well as patient and infection characteristics that may predict failure or reinfection after two-stage revision THA reimplantation.
MATERIALS AND METHODS
Study Design and Selection Criteria
This retrospective study was performed after institutional IRB approval. The authors reviewed the charts of all patients who had undergone two-stage THA revision at a single institution by a single surgeon between 2001-2011. All patients who had undergone a two-stage revision THA for periprosthetic infection were included in this study. A diagnosis of infection was made by the treating surgeon guided by the musculoskeletal infection society (MSIS) criteria for the diagnosis of PJI: positive joint fluid cultures, joint fluid cell count and differential, inflammatory markers (CRP and ESR) presence of a sinus tract, gross purulence observed at the time of surgery, and a positive histological exam for acute inflammation in tissues obtained during surgery. We identified 62 patients who were treated by a two-stage THA revision for periprosthetic infection by the senior author (DE) with a single surgical technique, utilizing an articulating antibiotic spacer fabricated intraoperatively with a customized mold.
Data Collection
Demographic and patient data were collected from hospital records for all patients including; BMI, age, gender and infection markers (CRP, ESR). Infection data were evaluated by collecting both preoperative and intraoperative cultures, and synovial fluid cell counts and differential. Perioperative complications were recorded from the patients’ charts. The overall preoperative medical status of the patients was evaluated by the Charlson comorbidity index (22). Failure of infection eradication was diagnosed based on both positive cultures and clinical diagnosis.
Surgical Technique
Aposterior-lateral approach to the hip (Kocher-Langen-beck) was utilized in all cases, with a joint fluid aspiration performed prior to the arthrotomy, followed by an extensive synovectomy and explantation of all implants. Perioperative antibiotics were held until the joint aspiration was completed. Endosteal membrane was removed from both the femoral medullary canal and the acetabulum, and was sent for culture and histology.
After verification that all remaining cement was debrided (if cemented implants were used), a ball tip guide wire was placed down the femoral canal. Sequential flexible reamers were used to ream the femoral canal until sufficient endosteal contact was created to remove all retained debris and create a bleeding bony surface. At that point we used our custom-made canal mold sizers (Fig. 1) to measure the femoral canal diameter (achieving a tight manual fit). Attention was shifted to the acetabulum where sequential reaming was conducted until a bleeding surface was identified. Then irrigation of both the acetabulum and the femoral canal was carried out using a pulse lavage device; first three liters of saline solution were used in the femoral canal, with a long nose tip, followed by one liter of bacitracin solution (33,000 units per liter). Subsequently, the entire soft tissue envelope and acetabular bony surfacewere irrigated with six liters of saline solution, followed by two liters of bacitracin solution (33,000 units per liter).
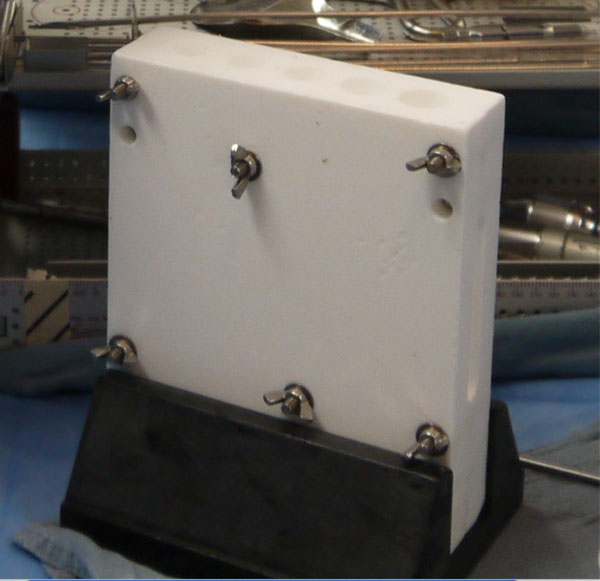
Custom-made canal mold sizers.
To conserve surgical time, we simultaneously fabricated the cement-tapered stem on the back table. Our specialized stem mold was used to produce the antibiotic femoral stem by coating a modular stem (S-ROM, Depuy, Warsw). The femoral head spacer was fabricated by covering a 22 mm CoCr head with a cement-molded spacer (fabricated from a surgical irrigation bulb syringe). Our protocol includes 80 grams (two packs) of polymethylmethacrylate cement (Simplex P; Stryker, Mahwah, NJ), premixed with 1 gram of tobramycin per 40 grams of cement. To this we added 4.8 grams of tobramycin powder, and 2 grams of vancomycin powder for a total of 4.4 grams of antibiotics per 40 grams of cement powder. For mixing we added a third bottle of monomer due to the added volume of the antibiotics. The cement was mixed and poured into the appropriate size tapered stem mold and bulb syringe mold (Fig. 2A, b). A femoral modular stemwas placed into the cement mold (Fig. 3), and a femoral head was placed into the syringe mold (Fig. 4). After full polymerization of the cement, the mold and bulb syringe were split and the stem and head were removed (Fig. 5A, b). The stem was placed appropriately into the femoral canal (Fig. 6). The hip was reduced and stability examined. After verification of desired anteversion angle the spacer was secured by adding cement into the medial calcar flare and coating the exposed body of the implant to provide adequate rotational stability. The added cement protocol included 40 grams (one pack) of polymethylmethacrylate cement (Palacos R+G; Zimmer, Warsaw, IN), premixed with 0.5 gram of gentamycin per 40 grams of cement. To this we added 2.4 grams of tobramycin powder, and 1 gram of vancomycin powder for a total of 3.9 grams of antibiotics per 40 grams of cement powder. The decision to use two different cements for each phase of the surgery is due to their different work time, Simplex is more liquid and easier to pour into the mold, and Palacos has more work time and easier to use for “free hand” molding.
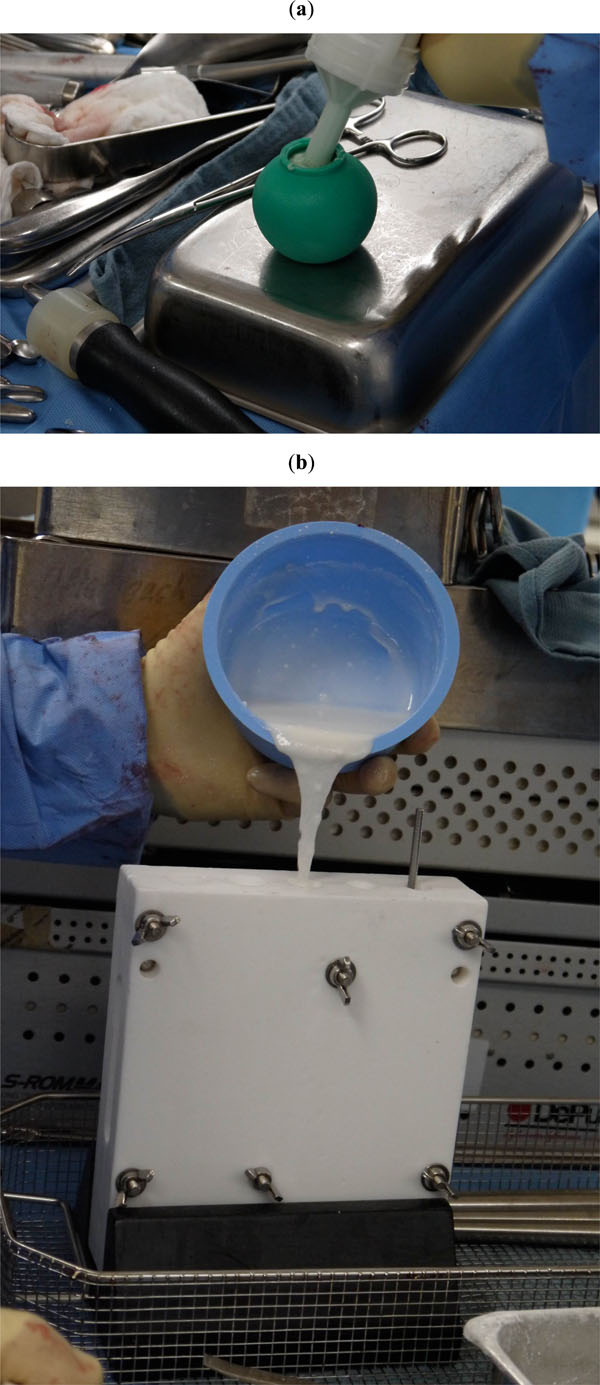
Cement was mixed and poured into the appropriate size tapered stem mold (a) and bulb syringe mold (b).

A femoral modular stem is placed into the cement mold.

A femoral head is placed into the syringe mold before the cement curs.
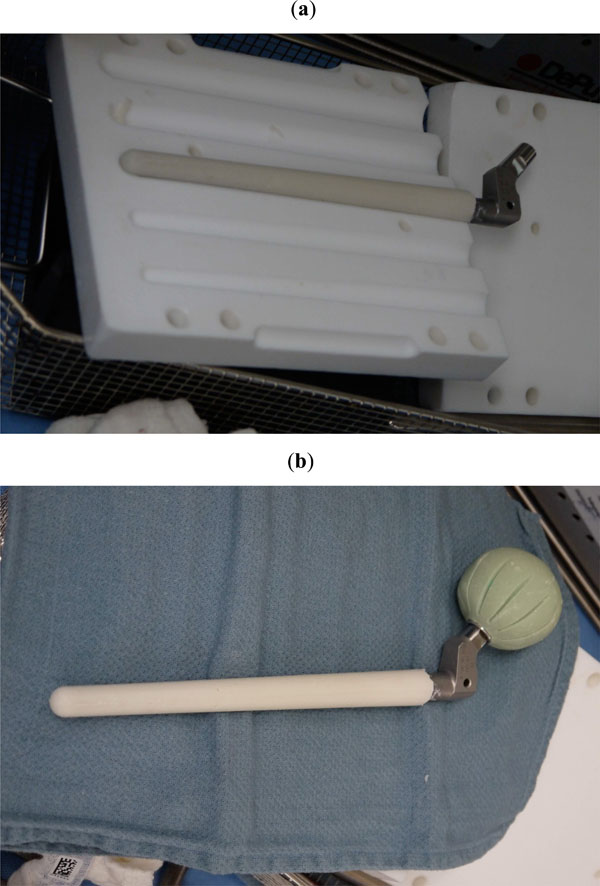
After full polymerization of the cement, the mold and bulb syringe are split and the stem (a) and head (b) were removed.
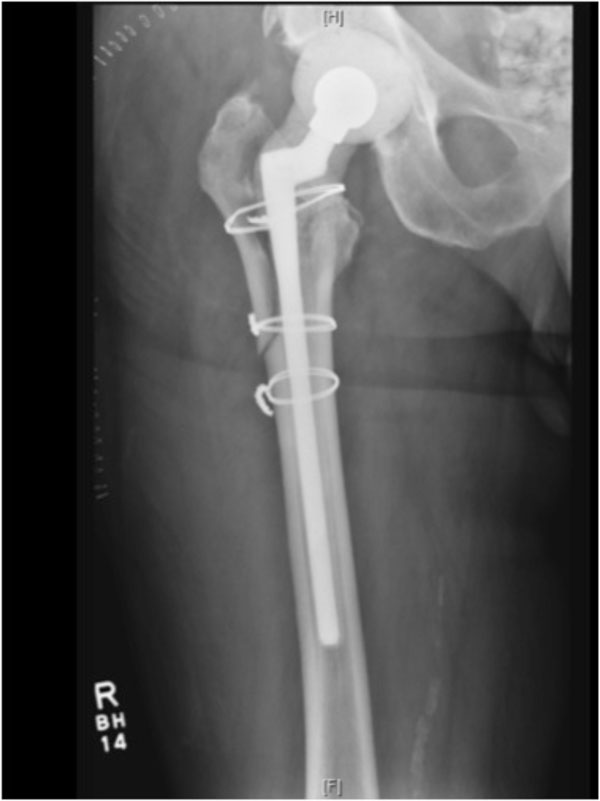
Postoperative radiograph after placement of a custom fabricated femoral mold.
Patients were allowed to partially weight bear on the operated leg with bilateral upper extremity support. Patients received culture specific intravenous antibiotic treatment for a period of at least 6 weeks under the supervision of an infectious diseases specialist. Patients were followed with serial inflammatory markers, CRP and ESR, during this period. Repeat arthrocentesis was done to monitor cell count and cultures after an antibiotic free period of at least 2 weeks. Surgical wound was monitored during office visits at 2 weeks and 6 weeks postoperatively, and 1 week prior to the planned 2nd stage reimplantation surgery. Patients were monitored for complications related to the procedure and treatment.
Reimplantation was considered between 3-4 months after the initial procedure if the hip joint aspiration had a negative culture and low cell counts, as well as descending trend of inflammatory markers. Revision surgery was completed and intraoperative tissue and joint fluid cultures and cell counts were taken.
We defined “successful two-stage revision” if no further surgical procedures were conducted during the follow-up period (e.g. irrigation and debridement, explant, Girdlestone, amputation, revision THA for non-infectious etiology); this was our primary outcome. We defined “eradication of infection” if intraoperative cultures at the time of revision surgery were negative and if the patient had no further infection related procedure (irrigation and debridement, explant, etc.) or a diagnosis of periprosthetic joint infection in the following year after the 2nd stage procedure; this was our secondary outcome.
Data Analysis
Categorical variables were analyzed using chi-square test and Fisher’s exact test. Continuous variables were analyzed using the t-test and Wilcoxon test. All analyses were conducted using SAS software, version 9.1.3 (SAS Institute, Carey, NC).
RESULTS
Our study cohort consisted of 62 patients who were treated by a two-stage THA revision for periprosthetic infection. One patient died from unrelated co-morbidity and five patients were lost to follow-up prior to completing the 2nd stage procedure. 56 patients were included in the final analysis. A Charlson comorbidity index score of 0 or 1 was found in 64% of the patients, while 36% had a score of 2 or more. The median length of time between the patient’s primary THA surgery and the first stage revision surgery was 6.1 years (range: 1 month - 35 years). 35 of these patients (63%) had more than one surgery on the infected total hip before antibiotic spacer placement and eight had 2, four had 3, and four had 4 or more surgeries prior to the 1st stage revision and antibiotic spacer placement at our institution, these surgeries were performed by the initial surgeon prior to presentation at our center (these surgeries included failed I&D and revision surgeries). Only 38 of the 56 patients had complete preoperative cultures compared to 56 with complete intraoperative cultures. Staphylococcus species predominated as the organism responsible for the infections, with 23.7% of the preoperative cultures positive for Staphylococcus species, and 26.9% of the intraoperative cultures positive for Staphylococcus species (Table 1). No postoperative fractures or dislocations of the customized hip spacer occurred among the study cohort.
Distribution of microorganism isolated from the hip at different time frames.
| Microorganism | Preoperative Culture (N=38) |
Intraoperative Culture on DOS Explant (N=52) |
Intraoperative Culture on Replant/Revision (N=51) |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| E. coli | 0 | 1 (1.9%) | 0 |
| Enterococcus | 0 | 3 (5.8%) | 3 (5.9%) |
| MSSA | 4 (10.5%) | 3 (5.8%) | 0 |
| MRSA | 1 (2.6%) | 5 (9.6%) | 1 (2.0%) |
| Streptococcus (other) | 6 (15.8%) | 6 (11.5%) | 0 |
| Micrococcus | 1 (2.6%) | 1 (1.9%) | 0 |
| Proteus mirabilis | 0 | 1 (1.9%) | 0 |
| Corynebacterium | 1 (2.6%) | 1 (1.9%) | 0 |
| S. Aureus/Peptosteptococcus | 2 (5.3%) | 0 | 0 |
| Streptococcus (b-hemolytic)/S. aureus | 0 | 0 | 1 (2.0%) |
| S. Aureus/Propionibacter | 0 | 0 | 1 (2.0%) |
| Staph (coagulase neg) | 2 (5.3%) | 6 (11.5%) | 3 (5.9%) |
| Bacillus sp. (not anthracis) | 1 (2.6%) | 1 (1.9%) | 1 (2.0%) |
| Propionibacter | 3 (7.9%) | 8 (15.4) | 0 |
| Pseudomonas | 1 (2.6%) | 0 | 0 |
| S. saccharolyticus | 0 | 0 | 1 (2.0%) |
| S. aureus/E. coli/Morganella | 0 | 0 | 1 (2.0%) |
| No growth | 16 (42.1%) | 16 (30.8%) | 39 (76.5%) |
| Missing | 18 (--) | 4 (--) | 5 (--) |
| Any S. aureus | 9 (23.7%) | 14 (26.9%) | 7 (13.7%) |
Joint fluid analysis revealed a median WBC count of 9.7 cells/μL (range 3.0-23.8 cells/μL) preoperatively. Prior to reimplantation median CRP values were 4.5 mg/dL (range 0.08-489 mg/dL) and median ESR values were 25 mm/hr (range 2-125 mm/hr).
At final radiographic follow-up, all X-rays were evaluated and signs of loosening were evaluated: subsidence, presence of a bony pedestal, lack of osseous integration, radiolucent lines around the stem. None of the reimplanted components was radiographically loose.
After a mean follow-up (after the 2nd stage revision) of 2.7 years, a successful 2nd stage revision procedure was performed on 48 (85.7%) of the patients in the final analysis cohort of 56 patients. Of the remaining 8 patients, 6 underwent Girdlestone, one was lost to follow-up after the 2nd stage reimplantation and one underwent only one stage due to chronic infection. Eradication of the infection was documented in 91.1% of the patients, and 80.4% of patients experienced both eradication of the presenting infection and a successful 2nd stage procedure (Table 2). A “worse case” scenario including the 5 patients that were lost to follow-up as failures would drop our success rate to 78.6% for a successful 2nd stage revision, 83.6% eradication of infection, and 73.7% of patients experiencing both eradication of infection anda successful 2nd stage procedure.
Patients’ outcomes among three end points: successful 2-stage revision, eradication of infection, and achieving both a successful 2-stage revision and eradication of infection.
| Outcome (N=56) | Frequency (N, %) | 95% CI |
|---|---|---|
| 2-stage reimplantation | 48 (85.7%) | 76.5% - 94.9% |
| Eradication of infection | 51 (91.1%) | 83.6% - 98.6% |
| 2-stage reimplantation + infection eradication | 45 (80.4%) | 70.0% - 90.8% |
We found no association between successful 2nd stage reimplantation and patient Charlson comorbidities index (p=0.70) (Table 3). Similarly, no association was found between successful 2nd stage reimplantation and pre-reimplantation levels of ESR (median difference 11.2 mm/hr, p=0.68), and CRP (median 1.5 mg/dL, p=0.48)(Table 4).
Association between patient variables, and a successful 2nd stage outcome.
| Variable | 2nd Stage Success (N=48) |
2nd Stage Failure (N=8) |
p-Value |
|---|---|---|---|
| N (%) | N (%) | ||
|
Sex Male Female |
22 (81.5) 26 (89.7) |
5 (18.5) 3 (10.3) |
.46 |
|
Charlson Score 0-1 ≥ 2 |
30 (83.3) 18 (90.0) |
6 (16.7) 2 (10.0) |
.70 |
|
OR culture* None/other MSSA/MRSA |
41 (85.4) 7 (87.5) |
7 (14.6) 1 (12.5) |
1.0 |
|
OR culture* None/other MRSA |
43 (84.3) 5 (100) |
8 (15.7) 0 (0) |
1.0 |
|
Secondary surgery prior to explant No Yes |
19 (90.5) 29 (82.9) |
2 (9.5) 6 (17.1) |
.70 |
|
Other infected joints No Yes |
44 (89.8) 4 (57.4) |
5 (10.2) 3 (42.9) |
.05 |
* Total <75 due to missing data.
* < 56 due to missing data.
Association between patients’ age, BMI, and joint fluid markers, with 2nd stage revision outcome.
| Variable | 2nd Stage Success | 2nd Stage Failure | p-Value | ||||
|---|---|---|---|---|---|---|---|
| N | Mean (SD) | Median (Range) | N | Mean (SD) | Median (Range) | ||
| Age | 48 | 61.9 (14.9) | 62.2 (20.4-84.9) | 8 | 65.0 (11.3) | 58.9 (54.7-83.2) | .57* |
| BMI | 35 | 31.5 ( 8.4) | 30.2 (19.0-48.0) | 5 | 32.7 (10.0) | 30.2 (23.8-47.6) | .77* |
| ESR at reimplantation | 42 | 26.5 (2.0-125.0) | 5 | 25.0 (10.0-113.0) | .68** | ||
| CRP at reimplantation | 43 | 4.0 (0.08-489) | 5 | 15.2 (0.17-28.5) | .48** | ||
| WBC before explant | 45 | 8.3 (3.0-23.8) | 8 | 11.1 (4.9-22.8) | .10** | ||
| PMN (%) before explant | 43 | 72.0 (8.8) | 71.4 (54.2-92.9) | 8 | 78.4 (9.6) | 74.1 (68.6-93.0) | .07* |
* t-test.
** Wilcoxon test.
We found a suggestion of an association between having another infected total joint replacement and a failed 2nd stage procedure (p=0.05) (Table 3). Although not reaching statistical significance, those with lower WBC and PMN values prior to explant appear more likely to have a successful 2nd stage procedure (p=0.10 and p=0.07, respectively) (Table 4).
DISCUSSION
Periprosthetic infection after total hip arthroplasty is a devastating complication that increases morbidity for the patient and cost to the medical system. Today, two-stage revision surgery, with a high-dose antibiotic-laden cement spacer, is regarded as the appropriate treatment protocol for chronic periprosthetic infections after THA with reported success rates of 80-90% [15, 16]. The ultimate goal of two-stage revision surgery is to achieve a lasting eradication of the infection and a durable THA reconstruction. Eradication of the infection is achieved by implant extraction, debridement and irrigation of the tissues, elution of antibiotics from the polymethylmethacrylate spacer, and administration of systemic microorganism-specific, intravenous antibiotics.
Successful outcomes tend to be reported in the literature more often than unsuccessful ones; treatment of peripros-thetic infection is no exception. Most studies report the success rate among patients who underwent a two-stage revision THA and do not include patients who were lost to follow-up or that did not reach the 2nd stage of the procedure, thus eliminating a sub group of patients with poor outcomes. The purpose of the study was to report the success rate of two-stage revision THA in a subpopulation of patients referred to a tertiary specialty hospital and to try to identify predictors of failure to eradicate infections and of poor outcome.
Our results are similar to other more current results reported in the literature. Biring et al. reported an 89% infection control rate after two-stage revision THA infection [17], and Wentworth et al. reported a success rate of 83% (success was defined as a negative culture take during the 2nd stage procedure) [18]. These reported results are similar to the 91.1% infection eradication rate reported in our cohort. Most studies do not examine the success of two-stage revision surgery and eradication of infection separately. We have subdivided our results in order to report a more meaningful success rate. Our combined success rate of both eradication of infection and long term survival of the two-stage revision surgery was 80.4%. In this group we do not include patients who were treated with suppression antibiotics (85.7% success of a two-stage revision THA was achieved if this subgroup was included) or patients who had resection arthroplasty (Girdlestone procedure). Parvizi et al. and Leung et al. [19, 20] showed a 75% and 79% infection control rate for methicillin-resistant Staphylococcus in two-stage THA with an antibiotic spacer, respectively. Berend et al. in a study of 202 patients treated with a two-stage procedure showed a 76% success rate in achieving infection eradication and a successful two-stage revision THA when they included the entire patient cohort, compared to an 83% success rate when only the subgroup of patients that underwent the second stage procedure were included [21]. Their data are similar to ours, and show that patients with more virulent organisms or higher comorbidities have a slightly lower success rate for two-stage revision THA.
Another important finding in our series was the absence of any antibiotic spacer fractures; spacer fractures are reported in the literature as a complication of two-stage revision surgery [17]. This emphasizes the importance of an endoskeleton to increase spacer strength and durability.
Over half of our cohort (63%) had more than one hip surgery prior to presentation at our institution; this reflects the complexity of the patient group. This was not found to be a significant predictor of failure. We did find that a history of another periprosthetic joint infection was highly predictive of failure of a two-stage THA revision procedure. This could point towards a patient group more susceptible to infection or harboring a more virulent organism. Practitioners should take this into account when counseling a patient with prosthetic joint infection and that has a history of a previous prosthetic joint infection in a different articulation.
Our study had a few limitations. First, our cohort is small and is derived from a single center. Second, due to the limited sample size, we were unable to construct a more complex analysis model to examine the multitude of variables among our patients, especially the influence of medical comorbidities. Despite these limitations our study represents a consecutive single surgeon experience with two-stage revision surgery for complicated chronic THA periprosthetic infection using a single treatment protocol, with most of the patients (63%) undergoing multiple hip surgeries prior to presentation.
The failure rate of simultaneous eradication of infection and successful two-stage revision observed in our series may be attributed to the specific patient characteristics typical of a referral center; such as multiple comorbidities and previous surgical failures. Factors affecting the high failure rate observed in our cohort may include a history of other previously infected prosthetic joints at any time during the patient’s past. We suggest that it is important to emphasize in current reports not only the infection eradication rate but also the total success of the two-stage revision surgery, which should include both the successful 2nd stage reimplantation of the THA prosthesis as well as eradication of the infection.
CONCLUSION
Patients with a history of other periprosthetic joint infections have a lower rate of a successful outcome of two-stage revision and should be counseled prior to the procedure on possible increased chance of treatment failure.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Supported in part by NIAMS P60 AR047782 and by the department of Orthopaedic Surgery, Brigham and Women's Hospital.


