All published articles of this journal are available on ScienceDirect.
Treatment Failure Among Infected Periprosthetic Patients at a Highly Specialized Revision TKA Referral Practice
Abstract
Deep infection is a serious and costly complication of total knee arthroplasty (TKA), which can increase patient morbidity and compromise functional outcome and satisfaction. Two-stage revision with an interval of parental antibiotics has been shown to be the most successful treatment in eradicating deep infection following TKA.
We report a large series by a single surgeon with a highly specialized revision TKA referral practice.
We identified 84 patients treated by a two-stage revision. We defined “successful two-stage revision” as negative intraoperative cultures and no further infection-related procedure. We defined “eradication of infection” on the basis of negative cultures and clinical diagnosis.
After a mean follow up of 25 months, eradication of the infection was documented in 90.5% of the patients; some had undergone further surgical intervention after the index two-stage procedure. Successful two-stage revision (e.g. no I&D, fusion, amputation) was documented only in 63.5% of the patients. We also observed a trend between presence of resistant staphylococcus (MRSA) (p=0.05) as well as pre-revision surgical procedures (p=0.08) and a lower likelihood of successfully two-stage revision.
Factors affecting the high failure rate included multiple surgeries prior to the two-stage revision done at our institution, and high prevalence of MRSA present among failed cases.
The relatively high rate of failure to achieve a successful two-stage revision observed in our series may be attributed to the highly specialized referral practice. Thus increasing the prevalence of patients with previous failed attempts at infection eradication and delayed care as well as more fragile and immune compromised hosts.
INTRODUCTION
Total knee arthroplasty (TKA) has been shown to be a reliable and cost-effective procedure, with expected survivorship of around 95% at 15 years [1-4]. Deep infection is a serious and costly complication of TKA, which can increase patient morbidity and decrease patient outcome and satisfaction [5-7]. With an incidence of deep infection between 1%-2% of all primary TKA, and even higher in revision cases, thousands of deep infections of TKA occur annually in the United-States [5-8]. Different treatment options have been described in the literature; two-stage revision with an interval of parental antibiotic treatment has been shown to be the most successful treatment in eradicating deep infection following TKA [7, 9-11]. Success rate of two-stage revision protocols has been shown to be around 90% and have become the standard of care in North America for chronically infected TKAs [9-5].
A variety of techniques to manufacture the intra-articular antibiotic cement spacer have been reported in the literature and good results have been described for both pre-manufactured and intraoperatively fashioned cement spacers [16-18]. Currently both static and articulating cement spacers are used during the interstage period, each with its reported benefits and complications [15, 19-21]. Static and articulating spacers serve as sources of local antibiotic delivery and maintain soft tissue tensioning. Articulating spacers have been described to help patients maintain a specified range of motion during treatment, which has been shown to facilitate the second stage surgery and improve final range of motion. Reports of fractures of static spacers and dislocation of articulating ones have been described as complications of such treatments [15, 19-21].
We report a large series of patients with deep infection following TKA managed by a single surgeon with a highly specialized revision TKA referral practice. The surgeon used a standardized surgical technique and treatment protocol, including an intraoperative fabricated static cement spacer constructed from a custom-made mold. Intraoperative fabrication of the cement spacer with a customized mold allows the surgeon the freedom of utilizing different cement types, choosing the added amount and type of antibiotics, and customizing the mold to the patient’s anatomy. We report the rate of infection eradication and successful two-stage revision, patient and infection characteristics that may predict failure or reinfection, and range of motion achieved after reimplantation.
METHODS AND MATERIALS
Study Design and Selection Criteria
This retrospective study was performed after institutional IRB approval from Brigham and Women’s Hospital investigation research board. The authors reviewed the charts of all patients who had undergone two-stage TKA revision at a single institution by a single surgeon between 2001-2011. All patients who had undergone a two-stage revision TKA for periprosthetic infection were included in the study. A diagnosis of periprosthetic joint infection (PJI) was based on joint fluid cultures, joint fluid cell count and differential, inflammatory markers, presence of a sinus tract, gross purulence observed at the time of surgery, and a positive histological exam for acute infection and inflamation in tissues obtained during surgery (the criteria used is aligned with current literature guidelines for diagnosis of PJI).
We identified 84 patients who were treated by a two-stage TKA revision for periprosthetic infection by the senior author (DE) with a single surgical technique, utilizing a static antibiotic spacer fabricated intraoperatively with a customized mold.
Data Collection
Demographic and patient data were collected from hospital records for all patients including; BMI, age, gender and infection markers including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). Infection data were evaluated by collecting both preoperative and intraoperative cultures, and synovial fluid cell counts and differential. Perioperative complications were recorded from the patients’ charts. The overall preoperative medical status of the patients was evaluated by the Charlson comorbidity index [22]. Failure to eradicate infection was diagnosed based on both positive cultures and clinical diagnosis.
Surgical Technique
A median parapatellar approach was utilized in all cases, with a joint fluid aspiration performed prior to the arthrotomy, followed by an extensive synovectomy and explantation of all implants. Perioperative antibiotics were held until the joint aspiration was completed. Endosteal membrane was removed from both the femoral and tibial medullary canals and was sent for culture and histology.
After verification that entire remaining cement was debrided, a ball tip guide wire was placed down the tibial canal. Sequential flexible reamers were used to ream the tibial and femoral canals until sufficient endosteal chatter was created to remove all retained debris and create a bleeding bony surface. At that point we used our custom-made canal mold seizers (Fig. 1) to measure both the tibial and femoral canal diameters. Then irrigation of both canals was carried out using a pulse lavage device; first three liters of saline solution were used in each canal, with a long nose tip, followed by one liter of bacitracin solution (33,000 units per liter) in each canal. Subsequently, the entire soft tissue envelope and bony surfaces were irrigated with six liters of saline solution, followed by two liters of bacitracin solution.
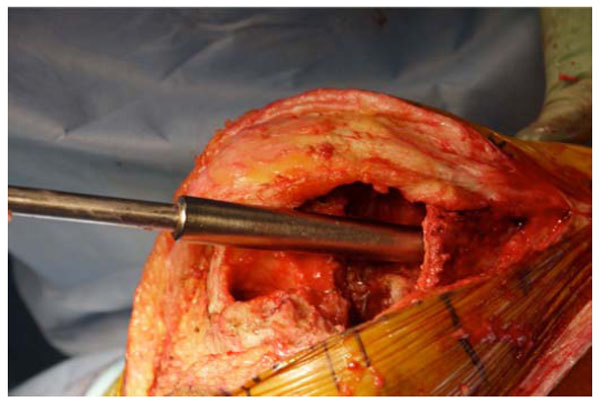
Tibial canal sizing with the costumed tapered canal seizers.
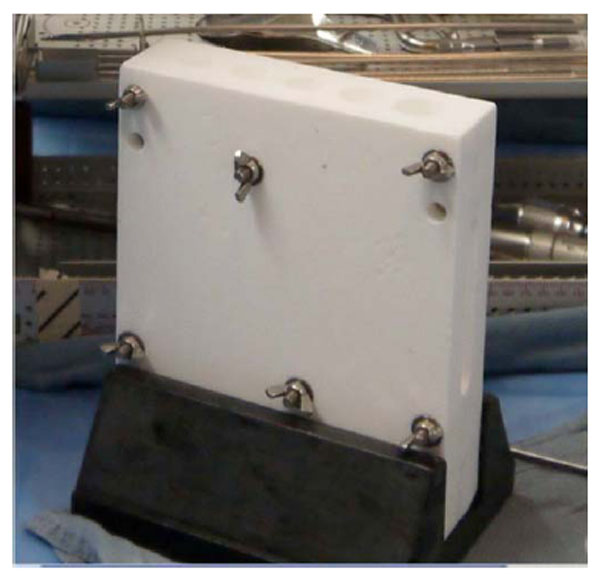
Antibiotic cement spacer costume mold.

Antibiotic cement being pored into the costume mold for preparation of an antibiotic spacer.

Costume cement spacers curing in the costume mold with Schanz pins inserted as a rebar for spacer strengthening.
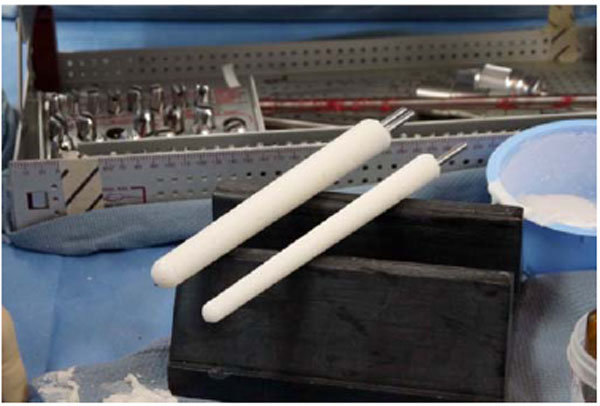
Prepared costume molded cement spacers for the femur and tibia canals.

Canal cement spacers placed in the femur and tibia and secured with 18 gage wires.
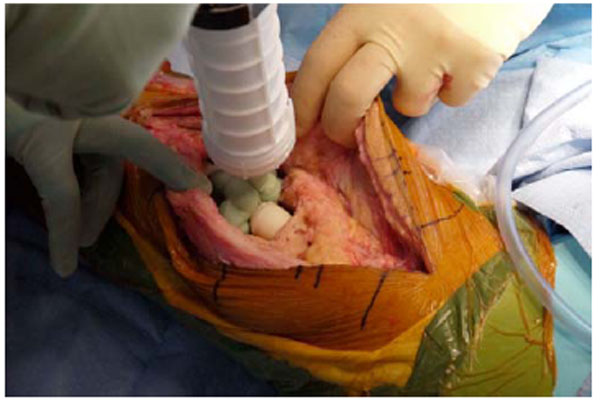
Antibiotic cement added to the knee joint to serve as an intraarticular spacer.
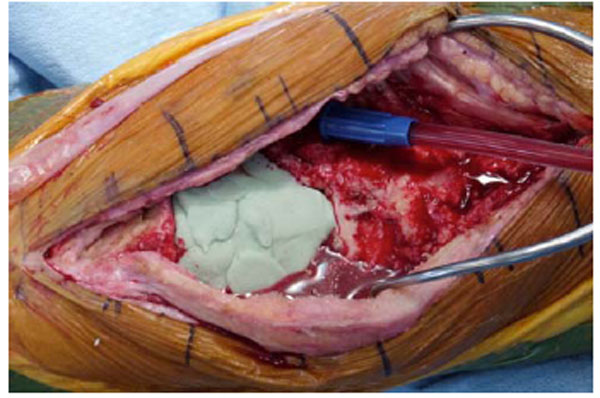
Tourniquet is deflated while the antibiotic cement spacer is hardening.
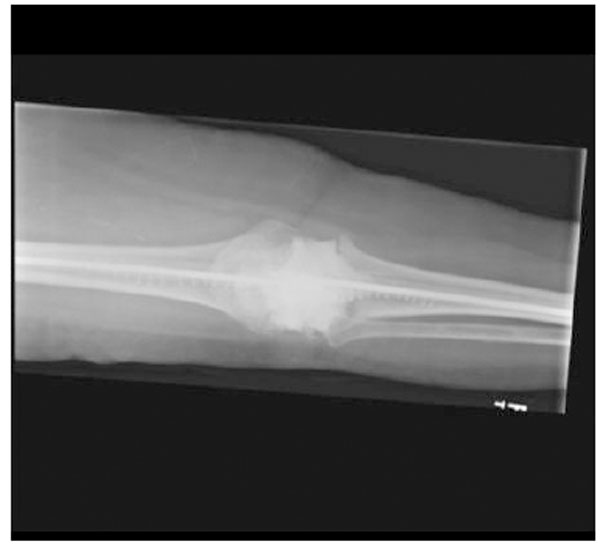
Anteroposterior X-ray of the intraarticular antibiotic cement spacer and costume molded canal spacers.
To conserve surgical time, we simultaneously fabricated the cement-tapered stems on the back table. Our specialized stem mold (Fig. 2) was used to produce the antibiotic canal spacers. Our protocol includes 80 grams (two packs) of polymethylmethacrylate cement (Simplex P; Stryker, Mahwah, NJ), premixed with 1 gram of tobramycin per 40 grams of cement. To this we added 4.8 grams of tobramycin powder, and 2 grams of vancomycin powder for a total of4.4 grams of antibiotics per 40 grams of cement powder. For mixing we added a third bottle of monomer due to the added volume of the antibiotics. The cement was mixed and poured into the appropriate size tapered stem molds (Fig. 3). A large threaded Steinmann pin was placed into each cement mold leaving 2 inches protruding beyond the mold (Fig. 4). After full polymerization of the cement, the mold is split and the stems are removed (Fig. 5). The stems were placed appropriately in the femoral and tibial canals, any excess length of the Steinmann pins was trimmed with a bolt cutter, and the overlapping pins were linked together with an 18-gauge cerclage wire (Fig. 6). The leg was placed in nearly full extension, with physiologic external rotation of the tibia. Three packs of 40 grams of polymethylmethacrylate each with premixed 0.5 grams of gentamycin (Palacos R+G; Zimmer, Warsaw, IN) were mixed with 7.2 grams of tobramycin powder, and 3 grams of vancomycin powder for a total of 3.9 grams of antibiotics per 40 grams of cement powder. For mixing we added a fourth bottle of monomer due to the added volume of the antibiotics. The cement was injected into the space created between the distal femur and the proximal tibia overlaying the Steinmann pins (Fig. 7). At this point, during polymerization of the cement, the tourniquet was deflated (Fig. 8).
Patients were allowed to fully weight bear on the operated leg with bilateral upper extremity support as long as the knee immobilizer was in place (Fig. 9). Patients received culture specific intravenous antibiotic treatment for a period of at least 6 weeks under the supervision of an infectious diseases specialist. Patients were followed with serial inflammation markers, CRP and ESR, during this period. Repeat arthrocentesis was done to monitor cell count and cultures after an antibiotic free period of at least 2 weeks. Surgical wound was monitored during office visits at 2 weeks and 6 weeks post operatively, and 1 week prior to the planned 2nd stage reimplantation surgery. Patients were monitored for complications related to the procedure and treatment.
Reimplantation was considered between 3-4 months after the initial procedure if the knee joint aspiration had a negative culture and low cell counts, as well a descending trend of inflammatory markers. Revision surgery was completed with Palacos R+G bone cement (Palacos R+G; Zimmer, Warsaw, IN), and intraoperative tissue and fluid cultures were taken.
We defined “successful two stage revision” if no further surgical procedures were conducted during the follow up period (e.g. irrigation and debridement, explant, fusion, amputation, revision TKA for non infectious etiology); this was our primary outcome. We defined “eradication of infection” if intraoperative cultures at the time of revision surgery were negative and if the patient had no further infection related procedure in the following year; this was our secondary outcome.
Data Analysis
Categorical variables were analyzed using chi-square test and Fisher’s exact test. Continuous variables were analyzed using the t-test and Wilcoxon test. All analyses were conducted using SAS software, version 9.1.3 (SAS Institute, Carey, NC).
RESULTS
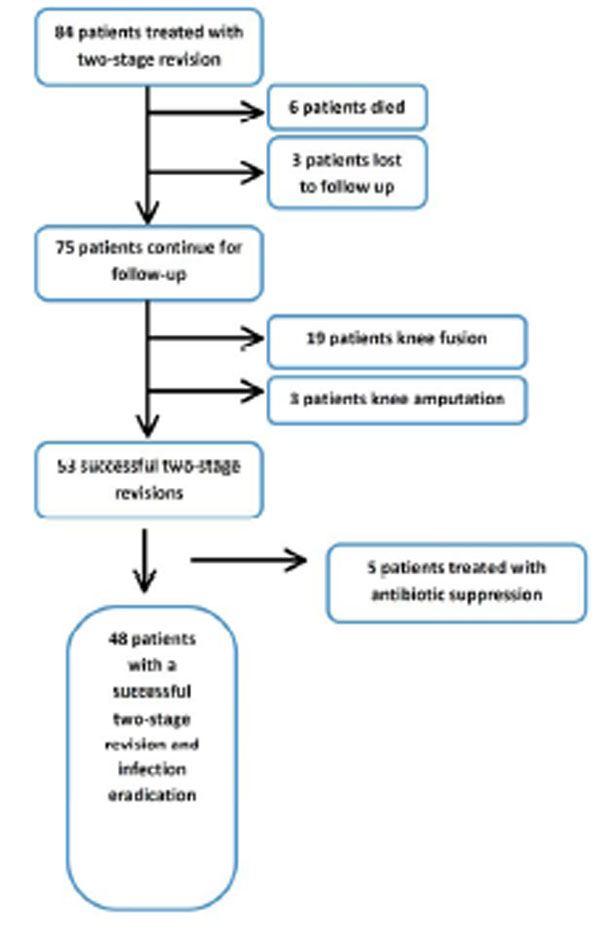
Our study cohort consisted of 84 patients who were treated by a two-stage TKA revision for periprosthetic infection. Six patients died and three patients were lost to follow up prior to completing the 2nd stage procedure. 75 patients were included in the final analysis, with a mean age of 67.3 ±12.0 (range 41.4-90.1), 38 males (50.7%), and 37 females (49.3%). Average BMI was 33.2 kg/m2 (range 22.6-59.1). A Charlson comorbidities index of 0 or 1 was found in 48% of the patients, 52% had an index of 2 or more. The median length of time between the patient’s primary TKA surgery and the first stage revision surgery was 3.1 years (range: 1 week - 26 years). Sixty of these patients (82%) had more than one surgery on the infected total knee before antibiotic spacer placement. Out of these twenty-two had 2 surgeries prior to antibiotic spacer placement, nineteen had 3, and nineteen had 4 or more surgeries prior to the first stage revision and antibiotic spacer placement at our institution. Two patients had undergone preliminary cement spacer at an outside institution (all of them underwent a repeat 1st stage procedure at our institution).
Only 38 of the 75 patients had complete preoperative cultures compared to 67 with complete intraoperative cultures. Staphylococcus species predominated as the organism responsible for the infections, with 34.3% of the preoperative cultures positive for Staphylococcus species (MSSA 21.1%, and MRSA 13.2%), and 41.8% of the intraoperative cultures positive for Staphylococcus species (MSSA 25.4%, and MRSA 16.4%) (Table 1).
Knee Joint Fluid Culture Results Taken in Clinic Before the Patient Underwent Explant and Antibiotic Spacer Placement and During the Procedure Itself
| Microorganism | Preoperative Culture N=38 | Intraoperative Culture N=67 |
|---|---|---|
| N (%) | N (%) | |
| E. coli | 4 (10.5%) | 2 (3.0%) |
| Enterococcus | 3 (7.9%) | 4 (6.0%) |
| MSSA | 8 (21.1%) | 16 (23.9%) |
| MRSA | 5 (13.2%) | 11 (16.4%) |
| Streptococcus (other) | 4 (10.5%) | 6 (9.0%) |
| Micrococcus | 0 | 1 (1.5%) |
| Corynebacterium | 0 | 3 (4.5%) |
| Peptostreptococcus magnus | 0 | 1 (1.5%) |
| E.coli/MSSA/P.mirabilis | 0 | 1 (1.5%) |
| Other | 3 (7.9%) | 0 |
| No growth | 11 (29.0%) | 22 (32.8%) |
| Missing | 37 (--) | 8 (--) |
Patients’ Outcomes Among Three End Points: Successful 2-Stage Revision, Eradication of Infection, and Achieving Both a Successful 2-Stage Revision and Eradication of Infection
| Outcome (N=75) | Frequency (N, %) | 95%CI |
|---|---|---|
| 2-stage reimplantation | 53 (70.7%) | 58.5 - 82.9 |
| Eradication of infection | 68 (90.7%) | 83.9 - 97.5 |
| 2-stage reimplantation + infection eradication | 48 (64.0%) | 48.7 - 79.3 |
Association Between Patient Variables, and a Successful 2nd Stage Outcome
| Variable | 2nd Stage Success (N=53) | 2nd Stage Failure (N=22) | p-Value |
|---|---|---|---|
|
|
|||
| N (%) | N (%) | ||
|
|
|||
| Sex | .56 | ||
| Male | 28 (52.8) | 10 (45.5) | |
| Female | 25 (47.2) | 12 (54.6) | |
|
|
|||
| Charlson Score | .19 | ||
| 0-1 | 28 (52.8) | 8 (36.4) | |
| ≥ 2 | 25 (47.2) | 14 (63.6) | |
|
|
|||
| OR culture* | .56 | ||
| None/other | 29 (60.4) | 10 (52.6) | |
| MSSA/MRSA | 25 (47.2) | 9 (47.4) | |
|
|
|||
| OR culture* | .49 | ||
| None/other | 41 (85.4) | 15 (79.0) | |
| MRSA | 7 (14.6) | 4 (21.1) | |
|
|
|||
| Secondary surgery prior to explant | .11 | ||
| No | 30 (56.6) | 8 (36.4) | |
| Yes | 23 (43.4) | 14 (63.6) | |
|
|
|||
| Other infected joints | .69 | ||
| No | 48 (90.6) | 19 (86.4) | |
| Yes | 5 (9.4) | 3 (13.6) | |
* < 75 due to missing data.
Association Between Patients’ age, BMI, and Joint Fluid Markers, with 2nd Stage Revision Outcome
| Variable | 2nd Stage Success | 2nd Stage Failure | p-Value | ||
|---|---|---|---|---|---|
| N | Median (Range) | N | Median (Range) | ||
| Age | 53 | 67.8 (41.4-90.1) | 22 | 69.9 (43.5-85.7) | .50* |
| BMI | 27 | 30.7 (22.6-48.9) | 17 | 33.7 (23.5-59.1) | .33** |
| ESR at reimplantation | 48 | 47.5 (4.0-124.0) | 19 | 64.0 (13.0-107.0) | .09** |
| CRP at reimplantation | 50 | 11.0 (0.4-251.0) | 19 | 14.8 (1.0-193.1) | .06** |
| WBC at reimplantation | 41 | 19,000 (9-132,000) | 12 | 25,600 (260-99,600) | .37** |
| PMN (%) at reimplantation | 41 | 86 (2-99) | 12 | 91.5 (16-96) | .27** |
* t-test.
** Wilcoxon test.
Joint fluid analysis revealed a median WBC count of 25,650 cells/mcL (range 43-366,000) preoperatively, and 21,400 cells/mcL (range 9-132,000) intraoperatively during the first stage procedure. Prior to reimplantation median CRP values were 11.8 mm/hr and median ESR values were 50 mg/dL.
Patients’ average range of motion in the most recent follow up visit was -2.1 (range: 0 - -15) degrees of extension to 88.7 (range: 0-120) degrees of flexion. Thirty-two (65.3%) of the 49 patients with gait evaluation had a normal gait (evaluated on clinical exam) during their last follow up visit. At final radiographic follow up, none of the reimplanted components were radiographically loose.
After a mean follow up of 25.1 months (range 12 month to 108 months), a successful 2nd stage revision procedure was performed on 70.7% of the patients with 5 patients needing continuous antibiotic suppression treatment. Eradication of the infection was documented in 90.7% of the patients, and the presenting infection was eradicated and a successful 2nd stage was completed in 64.0% of patients (Table 2). Out of the 75 patient cohort 53 patients had a successful 2nd stage reimplantation, 19 underwent further fusion, and 3 underwent amputation as the final surgical outcome (Flow Chart). Criteria for fusion included continued infection as well as compromised extensor mechanism and poor patient medical condition (“Fragile host”). Patients that had an unacceptable risk of persistent infection and poor soft tissue coverage as well as patients that were not candidates of extensor mechanism allograft were offered a knee fusion.
Twelve patients had become reinfected after the two-stage revision; reinfection was documented at an average of 14.6 months after the 2nd stage reimplantation procedure. Eight of the patients had the same organism cultured as in the first infection, and 5 patients were infected with a new organism. Six patients were managed with vancomycin, 3 patients were managed with cefazolin, 1 patient with nafcillin, 1 patient with linezolid, and 1 patient with penicillin. Out of the 12 patients that were reinfected, 5 were treated with suppression antibiotics, 4 underwent fusion after a subsequent period of antibiotic spacer placement, and 3 underwent amputation after failed attempts of limb salvage.
Out of 75 patients, 8 had a history of a prior infected total joint replacement (in shoulder, hip, or contralateral knee); of these 8 patients, 3 had fusion. Of the 19 patients that underwent knee fusion, 12 were done due to persistent infection and 7 due to extensor mechanism failure in light of previous infection and previous multiple surgeries done in outside hospitals prior to presentation to our center (Table 3).
We found no statistically significant association between successful 2nd stage reimplantation and patient Charlson comorbidities index (p=0.19) (Table 3). A trend was noted when we examined the association between the pre-reimplantation levels of ESR and CRP (p=0.09, p=0.06 respectively), and the chance of a successful 2nd stage reimplantation (Table 4).
We found a suggestion of an association between any surgical procedure prior to the 1st stage procedure and a successful 2nd stage procedure with eradication of the infection (Table 3). Although not reaching statistical significance, those without any prior procedures appeared more likely to have a successful outcome with eradication of infection (p=.08). Of those without a prior surgical procedure, 28/38 (74%) had a successful outcome with infection eradication, as contrasted with 20/37 (54%) of those with a prior surgical procedure. In addition, there was a suggestion that those who had a MRSA (resistant staphylococcus) infection were less likely to show infection eradication (p=.05).
DISCUSSION
Periprosthetic infection after total knee arthroplasty is a devastating complication that increases morbidity for the patient and cost to the medical system. Today, two-stage revision surgery is regarded as the appropriate treatment protocol for chronic periprosthetic infections after TKA. The ultimate goal of two-stage revision surgery is to achieve a lasting eradication of the infection and a durable TKA reconstruction. Eradication of the infection is achieved by implant extraction, debridement and irrigation of the tissues, elution of antibiotics from the polymethylmethacrylate spacer, and systemic, microorganism specific, intravenous antibiotics.
Our study had a few limitations. First we had a relatively small number of patients included in the cohort. Second, due to the small sample size of our study, we were unable to construct a more complex analyses model to examine the multitude of variables among our patients, especially the influence of medical comorbidities. Despite these limitations our study represents a consecutive single surgeon experience with two-stage revision surgery for complicated chronic TKA periprosthetic infection using a single treatment protocol, with most of the patients 82% undergoing multiple surgeries prior to presentation.
Many studies have shown the value of joint fluid analysis for the initial diagnosis of periprosthetic infection [23-25], but few have reported about the predictive value of joint fluid characteristics on the reinfection rate and failure of the two-stage revision surgery [24, 26, 27]. Both Ghanem et al. in a study with 109 patients, and Kusuma et al. with 76 patients, did not show any predictive value for ESR and CRP lab values taken between the first and second stage procedure in predicting persistent infection [26, 27]. This is in line with the results presented by Mortazavi et al. where ESR and CRP were not predictive for the outcome of PJI revisions [28]. However, the study by Mortazai et al. attributes this to a possible Type II error. Although all the above studies showed a decrease in both values between the first and second stage procedure in cases where the infection was controlled. In our study we similarly observed a decrease in the levels of ESR and CRP between the first and second stage procedure. We were unable to recommend any cutoff levels due to the small number of patients in our study. In order to achieve significance, a multicenter effort to create a large patient cohort with comparable treatment protocols is needed in order to achieve these results.
The utility of joint fluid cultures obtained prior to reimplantation is still debated in the literature. Ghanem et al. reported a high incidence of both false positive and false negative results. However they still recommended obtaining joint fluid cultures prior to second stage reimplantation [27]. Lonner et al. reported that joint fluid cultures taken prior to the second stage reimplantation were not useful in predicting persistent infection [23]. In contrast a study by Kurd et al. reported that patients who failed two-stage revision were 3.37 times more likely to have a positive culture for methicillin-resistant bacteria [29]. Tigani et al. reported in their study that in the face of positive intraoperative cultures recurrence of infection was 83% compared to 12.5% with negative intraoperative cultures [30]. Similarly, we observed a higher rate of successful two-stage revision in the absence of methicillin-resistant staphylococcus infection.
Patients who undergo an irrigation and debridement procedure prior to a two-stage revision have been shown to have a higher failure rate as demonstrated by Sherrell et al. [31]. In their study 34% of the patients who had a previous irrigation and debridement procedure failed a two-stage revision procedure for TKA periprosthetic infection. These data concur with our finding suggesting that patients who had undergone pre-revision surgical procedures had a lower likelihood of a successful two-stage revision.
The higher failure rate of two-stage revision observed in our series may be attributed to the highly specialized referral practice of the senior author, which draws patients that had failed prior interventions for failed and infected TKA as well as poor patient characteristics (“fragile host”). The high rate of knee fusion seen in our cohort can be attributed to the high number of previous surgeries and the condition of the knee and extensor mechanism among our patients upon presentation to our center. Factors affecting the high failure rate may include previous surgical treatment for PJI, 82% of the cohort had multiple surgeries prior to the two-stage revision done at our institution, and high prevalence of MRSA present among failed cases.
Future research in the field should be aimed at finding better diagnostic tools to aid us in our decision process when considering reimplantation for chronic periprosthetic infection.
CONCLUSION
Patients infected by the staphylococcus organism, and those who have undergone multiple procedures prior to the two-stage revision may have a lower rate of a successful two-stage revision with eradication of the infection.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.
GRANT SUPPORT
- Program for Research Incubation and Development, Dept Orthopedics, BWH.
- NIH P60 AR 47782.


