All published articles of this journal are available on ScienceDirect.
Cement-Implant Interface Contamination: Possible Reason of Inferior Clinical Outcomes for Rough Surface Cemented Stems
Abstract
Background:
Shape-closed cemented implants rely on a stronger bond and have displayed inferior clinical outcomes when compared to force-closed designs. Implant contamination such as saline, bone marrow and blood prior to cement application has the potential to affect the cement-implant bond. The consequences of implant contamination were investigated in this study.
Methods:
Fifty Titanium alloy (Ti-6Al-4V) dowels were separated into ten groups based on surface roughness and contaminant, and then cemented in polyvinyl chloride tubes. Push-out testing was performed at 1mm per minute. The roughness of the dowel surface was measured before and after the testing. The dowel surface and cement mantel were analyzed using a Scanning Electron Microscopy (SEM) to determine the distribution and characteristics of any debris and contaminants on the surface.
Results:
Contaminants largely decreased stem-cement interfacial shear strength, especially for rough surfaces. Saline produced the greatest decrease, followed by blood. The effect of bone marrow was less pronounced and similar to that of oil. Increasing surface roughness increased the interfacial bonding strength, even with contaminants. There was a non-significant increase in mean bonding strength for smooth surfaces with bone marrow and oil contamination. SEM showed that contaminants influence the interfacial bond by different mechanisms. More debris was found on rough samples following testing.
Conclusions:
The results of this study underscore the importance of keeping an implant free from contamination, and suggest if contamination does occur, a saline rinse may further decrease the stability of an implant. The deleterious effects of contamination on rough surface cement bonding were considerable, and indicate that contamination at the time of surgery may, in part, contribute to inferior clinical outcomes for rough surfaced cemented stems.
INTRODUCTION
Poly methyl methacrylate (PMMA) has been used clinically since 1938. Due to its biocompatibility and suitable mechanical properties, PMMA has been incorporated into bone cement to affix implants during joint arthroplasty. However, the loss of fixation at the metal-cement interface is the primary reason for clinical loosening in the case of cemented femoral or knee implants [1-3]. The aseptic loosening process initiates as debonding at the metal-cement interface [4] which can cause micro-motion leading to wear debris, cement fissures and fractures and ultimately to implant loosening.
The bonding strengths of cement and implant materials free from contamination have been well reported. Several factors have been identified that may affect bond strength including; antibiotics [5], porosity and pore distribution of the cement [6], stem geometry [7], stem material properties [8] and stem surface finish [9]. However, revisions where the stem appears not to have been fully bonded may be the result of contamination of the stem prior to, or at the time of implantation. Although researchers [9-1] have shown that the rough surfaced cemented stem can achieve higher interface bonding strength, other studies [12-14] indicated that smooth surface finish cemented Titanium stem achieved better clinical survivorship. But no clear explanation of this outcome has been given. Possible contaminants include bone marrow, blood or saline. These contaminants may have not have the same effect on bonding with different surface finishes. Few studies have investigated their effects on the implant-cement bond.
Bone marrow contamination may originate from yellow marrow within the medullary canal, and when present may alter the implant-cement bond [15]. Interfacial blood can influence the fixation of the implant by reducing the amount of interlock [16]. Saline is often present in theater for irrigation and cooling high speed instruments and could find its way onto an implant prior to cementing. Simulated body fluids can cause corrosion of Ti alloy and reduced hardness of surface oxides [17]. Increasing pH or decreasing protein concentration magnifies this corrosive effect. Iesaka et al. [18] have shown soaking in saline after implantation reduced the shear strength of the stem-cement interface. Stone et al. [19] demonstrated that the inclusion of organic material tends to weaken cement-metal interfaces.
While these studies have evaluated different contaminants at differing time points and using different methods, the objective of this research is to evaluate the effect of contaminants (bone marrow, blood, saline) on the bond strength of bone cement and Titanium alloy (Ti-6Al-4V) stems with different surface roughness using the same testing protocol. The null hypotheses relating to cement-implant interfacial bonding strength that were tested in the current study include: (1) strength is not influenced by surface roughness; (2) strength is not affected by contamination for smooth and (3) rough samples. Additionally samples will be qualitatively evaluated for evidence of differential mechanisms of failure.
MATERIALS AND METHODS
Two different surface finishes of titanium alloy dowels (25mm*12.5mm dia) were prepared by design to represent the surface finishes of existing commercial stems [7]. Each roughness group included 25 samples. The roughness (Ra) of the dowel surface was measured in accordance with ISO Standard 97 with a Surfanalyzer (EMD-5400, Federal Products Co., Japan). Four contaminants (Phosphate Buffered Saline (PBS), ovine marrow, ovine blood, and olive oil as a negative control) were prepared and heated to 37°C. Each contaminant was smeared on the dowel surface completely and uniformly approximately 4 minutes prior to implantation. The temperature will influence the contaminant viscosity; and contaminant viscosity will have an influence on a thickness of the contamination layer. The degree of contamination was standardized with respect to the area of the contamination applied. This method was chosen to minimize the variation of contamination level, to recreate a more clinic like contamination scenario, as well as to avoid unrealistic artefacts such as scratching which may occur if required to scrape contaminants to a particular thickness. Fifty samples were separated into ten groups (n=5 per group) based on surface roughness and contaminant.
Surgical Simplex P (Stryker Howmedica Osteonics, Limerick, Ireland) cement was manually mixed in an open bowl fashion until the PMMA powder was completely saturated with the liquid, and a homogeneous liquid was obtained (approximately 90 sec after mixing). Polyvinyl chloride (PVC) tubes (25mm long with 20mm internal diameter) were place on a flat titanium plate and filled with cement. Within the bone cement working period, which is between 3.5 min to 4.5 min following the initiation of mixing; the prepared titanium alloy dowels were placed in the dough-like consistency cement with a holding jig that allowed the stem to be centralized in the PVC conduit, to assure that the dowel was well centered. Another flat Ti plate was placed on top for 1 hour before being removed. Samples were then placed in PBS at 37°C in a thermostatically controlled incubator for 7 days prior to mechanical testing. The ends of each sample were lightly polished to remove the thin film of cement and push out testing was performed at 1mm per minute, on the MTS Bionix 858 testing machine (MTS Systems Corporation, Eden Prairie, MN), with a 25 KN axial-torsional load transducer (model number: 662.20D-05, MTS system corporation, Eden Prairie, MN, USA). The sample was placed on a support with a 15mm diameter hole (Fig. 1) allowing sufficient gap to minimize non-uniform stress distributions [20]. Statistical analysis of peak load data was performed using ANOVA with a 0.05 significance level using SPSS for Windows (version 13.0, SPSS Inc., Chicago, IL) followed by a Tukey HSD post hoc analysis.
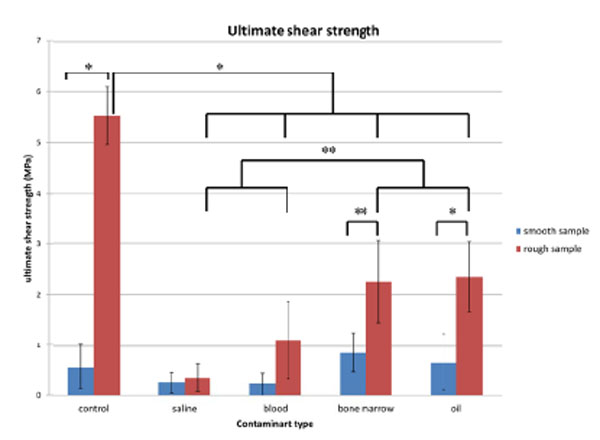
The ultimate shear strength for each surface preparation, error bars represent standard deviation, *= p<0.001, **= p<0.05.
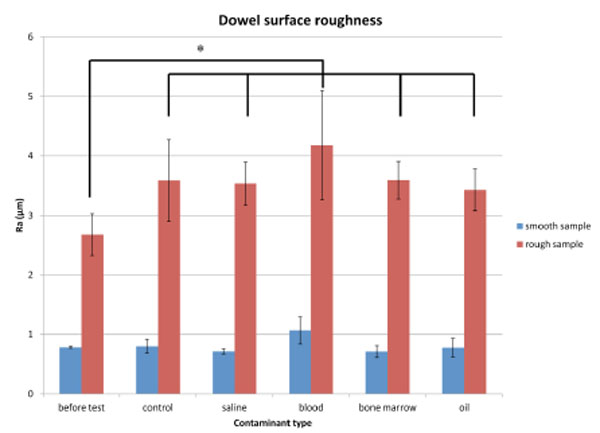
The Ra for each surface preparation for different bodily contaminants, error bars represent standard deviation., *= p<0.001.

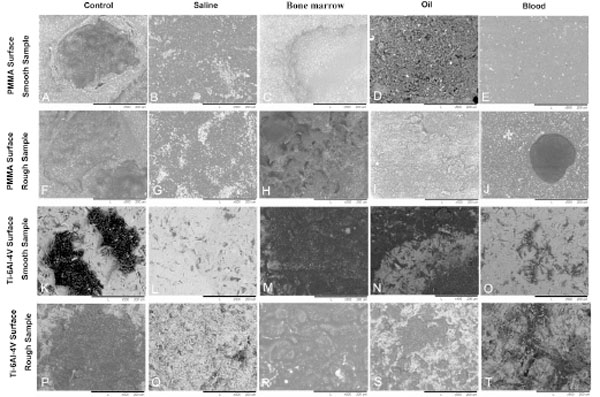
40x magnification SEM images of PMMA surface and dowel surface. (A-E) PMMA surface of each smooth sample group; (F-J) PMMA surface of each rough sample group; (K-O) Ti-6Al-4V dowel surface of each smooth sample group; (P-T) Ti-6Al-4V dowel surface of each rough sample group.
500x magnification SEM images of PMMA and dowel surfaces. (A-E) PMMA surface of each smooth sample group; (F-J) PMMA surface of each rough sample group; (K-O) Ti-6Al-4V dowel surface of each smooth sample group; (P-T) Ti-6Al-4V dowel surface of each rough sample group.
All samples were completely pushed out to allow further evaluation. The roughness of these Ti alloy dowels was measured with a Surfanalyzer profilometer (EMD-5400, Federal Products Co., Japan). The dowel surface and cement mantel were analyzed via Scanning Electron Microscopy (SEM) (TM-1000, Hitachi, Japan) to characterize surface debris and contaminants. Each sample was evaluated under low magnification to establish contaminant remnants across the entire surface, then low magnification images (40x) were taken at 3 different areas of the sample (top, middle and bottom). High magnification (500x) was used to focus on specific points of interest. Distance measurements were performed via the SEM. Qualitatively assessments reported here are based on author consensus.
RESULTS
Mechanical Testing
Rough dowels produced higher mean ultimate shear strength in both control and contaminated groups, when compared with smooth dowels under matched conditions (Fig. 1). Roughness was a factor that influenced bond strength for control, bone marrow and oil groups (p<0.001, p<0.05 and p<0.001 respectively), but this influence was not significant for the saline and blood groups.
All contaminants produced a mean value of ultimate shear strength lower than control, except for the smooth dowels with bone marrow or oil contamination. However, no statistical differences were found between the bone marrow and oil groups, or between saline and blood groups.
For rough dowels, there were differences in peak shear strength between control (5.52 ± 0.57 MPa) and all contaminated groups (p<0.001). Saline decreased the bonding to the greatest extent for rough samples. Blood (1.10± 0.75 MPa) and saline (0.36 ± 0.29MPa) groups had lower peak shear strength compared to bone marrow (2.25±0.81MPa) and oil (2.35±0.68MPa) groups (p<0.05).
For smooth samples, the ultimate shear strength was not significantly affected by contamination. The mean ultimate shear strength of the control group (0.57± 0.45 MPa) was lower than the bone marrow (0.87±0.38MPa) and oil (0.66±0.56Mpa) groups for smooth samples. The saline (0.26±0.21 MPa) and blood groups (0.23±0.23 MPa) showed decreased interfacial bonding between cement and smooth dowels, but as previously mentioned this did not reach significance.
Surface Roughness
Prior to push-out, smooth and rough sample Ra was 0.78 (±0.02) and 2.68 (±0.35) respectively. After testing, the surface roughness of the rough group increased for all conditions (p<0.05). However, for the smooth samples, the surface roughness was slightly lower following testing with saline, bone marrow and oil contamination, and higher under control and blood conditions (Fig. 2). Statistically, cementing did not affect post-test surface roughness for the smooth dowels. Furthermore, no relation between change in roughness and bonding strength of stem-cement interface was found.
SEM Imaging
PMMA debris was present on the Ti dowel surface after testing for the control groups (Figs. 3K, P, 4K, P). This was true to a greater extent for rough samples (Fig. 3P) than for smooth samples (Fig. 3K). The pattern of bone cement debris on Ti dowel surfaces matched well to the pattern of voids on the cement mantle surfaces. The white particles seen on PMMA and dowel surfaces, shown in Fig. (4), are suggestive of Barium Suphate [21, 22]. The PMMA surfaces of the control samples showed the presence of voids (Fig. 3A, 3F) of about 250 μm in diameter. Higher magnification revealed pores within the voids (Fig. 4A, F). Cracks were present at the edge of some of these voids. There was greater population of voids on the PMMA surfaces of rough samples (Fig. 3F) than smooth (Fig. 3A).
For saline groups, the macrography results showed a very smooth PMMA surface. SEM revealed a lack of voids in the PMMA mantle (Figs. 3B, G, 4B, G). Likewise no PMMA debris was present on the dowel (Figs. 3L, Q, 4L, Q). Instead, numerous bright structures were observed on both surfaces. These appear to be salt crystals remaining on the surface following the evaporation of the H2O portion of PBS. A greater number of these structures were found on rough samples and the distribution of these again matched the surface configuration for both the smooth and rough dowels.
For bone marrow groups, large holes were present on the mantle (Figs. 3C, H, 4C, H). Fig. (4C) shows the edge of one of these holes at high magnification. The surface configuration for rough samples was more complex, with porous structures (Fig. 4H) and dark areas on the dowel surface (Figs. 3M, R, 4M, R) suggesting PMMA or marrow contamination.
For oil contamination, the large dark area (Figs. 3D, I, N, S, 4D, I, N, S) with surrounding ring shaped structures were seen in these groups. Higher magnification images (Fig. 4N, S) suggest residual PMMA.
Blood was observed macroscopically on both cement and dowel surfaces (Figs. 3O, T, 4O, T). SEM imaging revealed cracks on the PMMA surface (Figs. 3E, J, 4E, J). White fragments on the dowel surface are likely PMMA particulates. Rougher surfaces appeared to retain more blood (Fig. 4T), which is supported by previous research [21]. A small number of voids and PMMA particulates were found on both surfaces.
DISCUSSION
Stem geometry, material selection and surface treatment play important roles in both cemented and uncemented hip implant design [9]. Due to the viscoelastic properties of PMMA, a polished force-closed design can bear some implant subsidence. However, the bonding strength of the shape-closed design implant is depended on the mechanical interlock at the cement-stem interface and will have a rough surface. The choice of implant design becomes more complicated when contaminants, such as saline, bone marrow and blood may bring about incomplete bonding between metal and PMMA, altering the intended design performance. The results of this study show that contamination can have considerably negative effects on the strength of this bond for rough surfaces.
Surface Roughness
Shear strengths were higher for control samples of higher surface roughness, which correlates to existing research [9]. Increasing implant surface roughness may allow for increased surface area in contact with cement as well as increasing the depth of the interdigitation and mechanical interlocking effect. This result was demonstrated by Walsh et al. [9], and in the current study it was shown that the bonding between cement and rough dowels stood firm even after failure. The energy required to break the PMMA free increased the peak loads of failure. Therefore, the bonding strength increased with roughness.
For rough samples, damage caused by breaking the micromechanical interlock, adherence of PMMA debris and, to a lesser degree, contaminants such as blood, led to increased roughness following testing. For smooth samples, the Ti surface roughness was not changed significantly, indicating that there was little PMMA debris or contaminants adherent. The presence of PMMA wear debris may have a significant influence on clinical outcome.
Pores were a common feature close to the interface of the Ti dowel. These pores are likely to have been involved with the initiation or propagation of the failure [25]. The increased fragmentation of the PMMA mantle could also play a role in long term loosening by acting as a grinding material. These particles are known to induce biological reactions with osteolytic consequences and are likely to contribute to implant failure [22-24].
Contamination
The saline groups showed the lowest bond strength, followed by the blood groups, while the effect of bone marrow was not different to oil. SEM results suggest that contaminants such as saline and blood form a layer on the metal-cement interface and reduce the amount of mechanical interlock; other contaminants such as bone marrow and oil produced unusual features suggesting the influence of more complex lipid-monomer interaction.
For the saline groups, no voids or PMMA debris were found on the PMMA or dowel surfaces, correlating to the low strength and energy required to break the bond between metal and cement [26-28]. The low values might be influenced by saline which forms a salt crystal layer, weakening the mechanical interlock between the cement and dowel. In addition, the crystals may act as fine particles, decreasing friction.
As would be expected, roughened samples retained more blood than smooth [21]. Again the small number of voids and PMMA particulates relates to the low bonding strength of the interface [26-28]. The large area occupied by blood on the dowel surface minimized the amount of interlock that could be achieved between the dowel and cement [16]. It should be noted that blood contamination may lead to non-bonding conditions for smooth samples as well. Although interface bonding strength is not essential factor for a force-closed implant, this contamination may also play a role in Biofilm formation, although this is beyond the scope of the present study
Oil was expected to cause a great decrease in bond strength and was included as a negative control. However the monomer portion is a strong lipid solvent and this interaction can explain many of the differences in bonding seen between groups. Arnold et al. [29] and Hailey et al. [30, 31] have investigated the influence of storage media on cured PMMA samples and have found that water is a stronger plasticizer than lipid and suggest that lipids may aid in the elution of unreacted monomer (a known plasticizer). This also supports our biomechanical results. Oil contaminated groups showed an interesting pattern with a ring surrounding structure indicative to the edge of an oil drop. There appears to be a great deal of residual PMMA along this edge that would suggest improved bonding at this area.
The large gaps seen in the PMMA mantle of the bone marrow group suggest the presence of marrow during curing. The afore mentioned interactions between the monomer and lipids are likely to have played a role in the formation of the complex structures seen in the SEM results.
Although the differences in push out loads were not significant for the smooth group the trend was apparent. The ultimate shear strength for the smooth control group was lower than that of the smooth bone marrow group. This was unexpected and prompted additional investigation. Another 6 smooth control group samples were tested and the results were not statistically different (p>0.05) and nearly identical. These results are also in line with other studies [18, 32]. With the validation of the non-contaminated results, the explanation must lie in the specific attributes of the contaminant and its interactions with PMMA and Ti. With rough samples, surface features can alter surface contact simply by retaining contaminants that later block physical access to crevices and thereby limit mechanical interlock. In the absence of significant surface features, the monomer’s ability to solubilize bone marrow and the lipid role in leaching of residual monomer are likely to have a greater influence in determining the interfacial strength. Coating the Ti dowel with a lipid layer may encourage more intimate contact. As oil is combined with the monomer during dowel insertion it may aid in wetting the Ti and encourage interdigitation into very small surface features. This could also explain the improved bonding around oil droplets in the rough group.
Contamination of the implant is difficult to avoid at the time of surgery. For force closed (polished) systems, secure bonding is less crucial, with a small amount of implant subsidence common and not detrimental to implant survival. Therefore further weakening of this bond has minimal impact. However, for shape closed systems (roughened) a small amount of motion generates PMMA wear debris which induces osteolysis and act as grinding material. If this contamination is currently occurring intra-operatively it would have less effect on force closed implants and may explain the better long term clinical outcome of force-closed design Ti alloy stems [12-14].
LIMITATIONS
This study is not without limitations, one of which is the reproduction of implant geometry. Compared to a tapered design, the uniform geometry does not tolerate any increase of tensile strains in the cement mantle. A tapered stem would convert shear forces to compression of the cement mantle [33]. Therefore, this test represented the worst case scenario for the stem-cement interface. One commercial package of cement was used for each group. A standard mixing technique was used to minimize variation; however it is still possible that cement preparation variability influenced results. The structures seen on PMMA and dowel surfaces in every group closely resemble Barium Sulfate, a radio-opaque additive of bone cement. The appearance of these particles is similar to those presented by Kuhn and Kurtz [34, 35]. However no component analysis or quantitative analysis has been applied.
Test results show the shear strength between titanium material and bone cement, but it may not stimulate the mechanical situation of cemented femoral head and tibial components in knee replacement, except the stem portions of tibial and femoral components when present. Although well established as a bone surrogate in this type of research [18, 30, 36], PVC tube may alter the peak temperature of the curing cement when compared to bone with active circulation. Most clinical implants do not fail by single exposure of high forces. Instead, repetitive exposure of low forces leads to failure over time. Although large differences were found between groups here showing that the study was effective at detecting differences, future work would do well to supplement this data with fatigue results. As with all in vitro laboratory studies, the test conditions do not replicate in vivo conditions. Testing in PBS at 37℃ would not ideally simulate these conditions but may have closed the gap.
CONCLUSION
The first null hypothesis that surface roughness does not influence bonding strength was rejected. Rough cemented samples were considerably stronger than smooth samples. The second null hypothesis was accepted. For smooth samples contamination did not significantly alter the interfacial bonding strength. The third null hypothesis was rejected. For rough samples, saline weakens the interface to the greatest extent, followed by blood. Areas of preferential bonding were seen around oil droplets; and with smooth samples, a trend of improved properties was seen in the presence of lipid based contaminants.
While it was true that contamination weakened the bond for rough interfaces, smooth interfaces were not affected. It should be noted that increasing surface roughness dramatically improved the load carrying capability of the implant-cement interface even in the presence of contaminants.
It is important to bring this knowledge into the clinical setting, to be aware of the potential detrimental effects that can be brought about by contact of pre cemented surfaces with surrounding tissues, especially for stems with roughened surfaces, or shape-closed designs. If contact does occur it is important to know that rinsing with saline will not improve, but may further decrease, the properties of the implant-cement bond. It is also notable that smooth surfaces did not generate PMMA debris, and there was no decrease in bonding strength with contamination. Rough samples were more susceptible to contamination and this contamination at the time of surgery may, in part, explain the inferior long term clinical outcomes with this type of cemented stem.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


