All published articles of this journal are available on ScienceDirect.
One-Stage Revision Arthroplasty for Infected Hip Replacements
Abstract
Infection remains a serious complication after total hip arthroplasty (THA) and is a leading cause of hip revision surgery. It is currently accepted that removal of the prosthesis is essential to curing an infection when facing chronic PJIs with prosthesis loosening. In order to avoid the disadvantages of a two-stage approach, some authors have proposed a one-stage hip revision for the treatment of hip prosthesis infection in selected patients using not only antibiotic-loaded cemented components but also cementless implant. In the case of a one-stage procedure, the patient is exposed to a single major procedure and therefore lower cumulative perioperative risk. A functional prosthesis replacement is completed without exposure to the complications associated with spacers. In addition, there are also benefits both financially and in terms of resource allocation.
INTRODUCTION
Infection remains a serious complication after total hip arthroplasty (THA) and is a leading cause of hip revision surgery. Despite correct surgical techniques and antibiotic prophylaxis, reported infection rates after THA remain between 1 to 3% [1, 2]. Up to approximately 30,000 THA are implanted annually in our country. Taking into account a 3% infection rate, a total of 3,000 cases per year of hip prosthesis infection are to be expected. Early diagnosis and treatment are mandatory to prevent an infection from becoming chronic and the corresponding hip replacement, which leads to higher morbidity and mortality as well as increased sanitary costs [3, 4].
Acute post-surgical prosthetic joint infection (PJI) can be treated correctly with surgical debridement and proper antibiotic therapy [5-8]. However, chronic PJIs imply high rates of relapse when treated with this strategy alone, even when antibiotic therapy is prolonged for months or even years [9, 10], and prosthesis removal is ultimately required [11]. The different response to treatment between acute and chronic PJIs may be related to the extension and maturity of the biofilm which covers the prosthesis. In view of the fact that surgical debridement in acute PJIs takes place before the biofilm has been formed and become stable, it is reasonable that debridement should also be more successful in this case.
It is currently accepted that removal of the prosthesis is essential to curing an infection when facing chronic PJIs with prosthesis loosening [12, 13]. Currently, two-stage hip revision is advocated for in the treatment of chronic hip prosthesis infection [14, 15]. This strategy involves a first stage which consists in surgical debridement, prosthesis removal, implanting an antibiotic-loaded cement spacer and specific antibiotic therapy; the second stage takes place after the infection has been eradicated and consists in removing the hip spacer and implantation of the definitive prosthesis. However, this strategy has some drawbacks: its long duration delays the patient's recovery; it implies a high social and economic cost and is not exempt from complications [16]. The reported failure rates after two-stage hip revision range from 5 to 18% [17, 18].
In order to avoid the disadvantages of a two-stage approach, some authors have proposed a one-stage hip revision for the treatment of chronic hip prosthesis infection using not only antibiotic-loaded cemented components [13] but also cementless implants [16].
PATIENT SELECTION
It has been recommended that candidates for a one-stage hip revision meet the following criteria [13]: 1) absence of immunosuppression, 2) absence of clinical signs of active infection, 3) absence of fistulae, 4) absence of major soft tissue defect compromising wound closure and/or bone defect affecting implant stability, and 5) infection caused by low-virulence microorganisms.
PATIENT MANAGEMENT
Clinical Data
Patients typically report hip pain for more than two weeks, which may or may not be accompanied by local inflammatory signs. Symptoms or signs that may be found during physical examination include groin or knee pain, pain in response to motion exercises, inability to bear weight, swelling, erythema, pain with external rotation and flexion or even fistulae, despite the fact that these are more commonly found in acute PJIs. The presence of a cutaneous fistula is indeed a pathognomonic sign of infection. It is mandatory to perform a proper differential diagnosis that includes aseptic loosening.
Multiple complementary tests are available, but none of them are conclusive in and of themselves. Thus, the final diagnosis is usually obtained from the combination of a complete clinical history, an exhaustive physical examination, laboratory tests and image findings.
Laboratory Tests
Some laboratory markers suggest the presence of infection in a painful prosthesis. However, no laboratory parameter is pathognomonic of infection. Despite the fact that elevated values of white-blood cell count, C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) have been reported during PJI, these levels can vary widely [19]. In fact, there are no studies to determine which cutoff values may predict prosthetic infection. Greidanus et al. studied 151 patients who underwent revision of knee prosthesis with a pre-operative diagnosis of aseptic loosening. They performed intraoperative cultures and considered infection when 2 or more samples were positive for the same microorganism. Pre-operative laboratory findings were recorded and analyzed in correlation with the final diagnosis (septic or aseptic loosening). They suggested that CRP and ESR levels higher than 1.35 mg/dL and 22.5 mm/h respectively should lead to the suspicion of prosthetic infection.
Image Findings
Plain radiology is the first technique used for the assessment of a painful prosthesis, and is able to identify the cause of pain in 25% of cases since it can diagnose fractures, dislocations and heterotopic bone calcifications (Fig. 1).
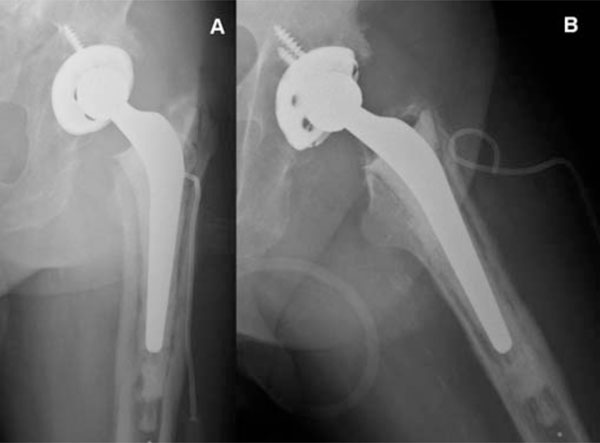
Posteroanterior (A) and axial (B) view x-ray imaging of pelvis showing left hybrid total hip arthroplasty (non-cemented cup, cemented stem).

The figure presents a patient with bilateral prosthesis and confirmed infection of the left hip. The leukocyte scintigraphy images (A: anterior, B: Posterior) show focal deposits in the left coxofemoral joint with fistulous trajectory towards the outer side of the thigh, inconsistent with the marrow scintigraphy (C: Anterior, D: Posterior), suggesting active infection (big arrows). Note that marrow scintigraphy findings are consistent with the leukocyte scintigraphy in the non-infected right hip prostheses (small arrows).
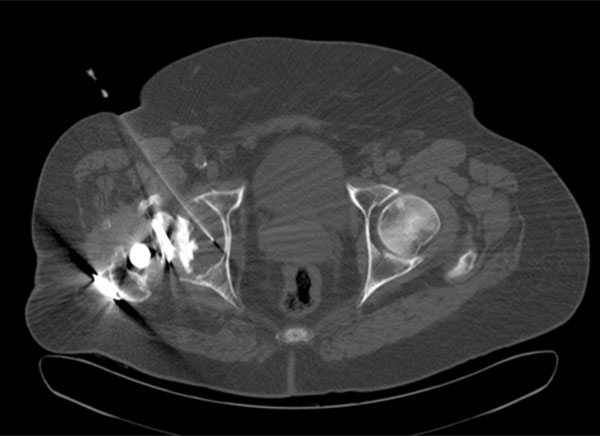
An 79-year-old man with surgical confirmation of infected right hip prosthesis (Staphylococcus epidermidis). Axial CT image allowed confirming placement of the needle in the middle of the prosthetic fluid. Positive cultures to Staphylococcus epidermidis were obtained on CT-Aspiration.

Intraoperative image showing longitudinal Wagner’s osteotomy in the femoral diaphysis.
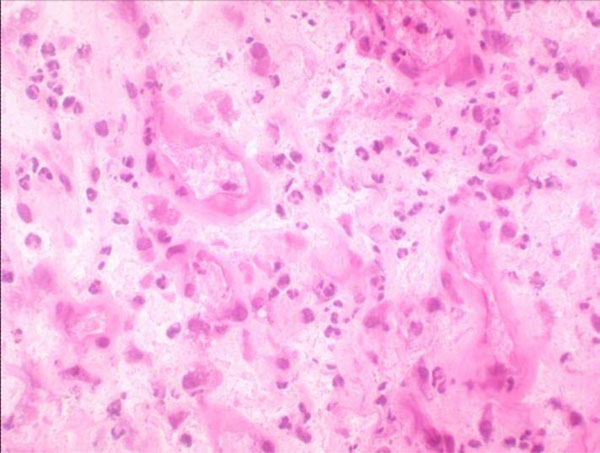
Hematoxylin-eosin staining high-power microscopic field (400x) in which ≥5 neutrophils are found.

Posteroanterior (A) and axial (B) view x-ray imaging of pelvis showing left cementless modullar revision arthroplasty after 3 years of follow-up.
However, in most patients, the complication is due to septic or aseptic loosening, and this usually requires other imaging tests to determine their aetiology [20]. Computed tomography (CT) and magnetic resonance have a diagnostic limitation in these patients due to the presence of artefacts created by the metallic components of the prosthesis. The combination of bone scintigraphy (BS) and 99mTc-HMPAO-labelled leukocyte scintigraphy (LS) is the most widely used imaging technique for the differential diagnosis of aseptic and septic loosening, providing a high sensitivity for detecting PJIs (Fig. 2).
However, the specificity of LS is relatively low due to bone marrow activation when in contact with the prosthesis, in which case a focal deposit of labelled leukocytes falsely leads to the suspicion of infection of the prosthesis [20, 21]. It has been reported that 99mTc-colloid bone marrow scintigraphy (MS) may be useful for distinguishing an activated bone marrow from leukocyte deposits due to active infection [22, 23]. Further studies have been recently reported using other radiotracers including FDG-PET, 99mTc-ciprofloxacine and scintigraphy with anti-granulocyte antibodies [24, 25]. Nevertheless, experience with these agents is limited and further studies are needed to asses their usefulness.
Preoperative Microbiology
Pre-operative identification of the infecting microorganism is of paramount importance in the treatment protocol for chronic PJIs, since it enables selection of the most appropriate antibiotic treatment.
Culture of the synovial fluid obtained through hip aspiration is a useful technique not only to confirm the presence of microorganisms, but to identify the particular microorganism responsible for the infection. However, it is technically difficult to perform and is not exempt from either complications or false negative results. The technique is generally performed under fluoroscopic X-ray guidance. The position of the needle can be confirmed by arthrography. In the past few years several articles have appeared on the usefulness of CT imaging (Fig. 3) in the evaluation of painful infection at the site of hip prosthesis before surgery [26].
However, joint aspiration has proven to have a broad range of sensitivity values and the frequency of dry-tap cases is not insignificant. In such dry-tap cases, percutaneous interface biopsy is a useful test for pre-operative isolation of the infecting microorganism [27].
Surgical Procedure
The patient is placed in a lateral position. Removal is always performed through pre-existing incisions. We routinely perform Hardinge’s direct lateral approach, but have sometimes chosen the Watson-Jones modified by Röttinger approach as well as the posterolateral approach. When facing a difficult removal of the implant, a major trochanter osteotomy is performed using the Wagner technique with fenestrations in the anterior femoral diaphysis when necessary (Fig. 4). Necrotic tissue is excised and the
wound is washed out with 10 litters of saline serum using either a high-pressure jet system or a conventional low-pressure system using bottles with no significant differences in both techniques [28].
Intraoperative Microbiology and Histology
Samples for the histological study were obtained from the periprosthetic membrane around the fracture [29, 30]. The samples were then fixed with formalin and embedded in paraffin; 4-μm sections were cut and stained with hematoxylin-eosin.
The Pathology Department at our hospital follows Mirra’s criteria (adapted by Feldman) [31, 32], according to which a sample is considered positive for infection when ≥5 neutrophils per high-power field (400x) are found in at least five separate microscopic fields (Fig. 5).
Samples for the microbiological study are always taken before the administration of antibiotic prophylaxis. At the time of prosthesis removal, at least six periprosthetic samples from different sites are submitted to the laboratory for culture.
Liquid samples aspirated from the surgical site with a sterile syringe are immediately inoculated into Batec 9000 Blood Culture Systems (Becton Dickinson Diagnostic Instruments, Sparks, Maryland) and incubated for five days [33]. Positive flasks are subcultured in aerobic and anaerobic agar media. Swab samples are obtained by passing a sterile swab (Delta-lab invasive sterile eurotube collection swab with Stuart transport medium; Rubí, Catalonia, Spain) over the areas of tissue, bone or fluid that are suspected of being infected. Solid periprosthetic tissue samples are immediately placed into a separate sterile universal bottle. Solid tissue samples and swab samples are cultured in both aerobic and anaerobic agar media and in thioglycolate broth enriched with vitamin K and hemin and are incubated for ten days. Positive cultures are sent for microorganism identification and sensitivity testing.
Follow-Up and Evaluation
After discharge, patients are seen monthly while they continue antibiotic treatment. Later, they have follow-up visits every six months for a minimum of 24 months. At each visit, clinical response and adverse events are recorded. Outcome is classified as follows after the final visit: 1) cure, when the patient presents no local signs of inflammation and CRP remains below 1mg/dl; 2) failure, when these criteria are not met. At the final visit, functional results are determined according to the Merle d’Aubigne scale and the Harris Hip Score.
DISCUSSION
Chronic infection remains a serious complication of total hip arthroplasty (THA). Several techniques have been used with the following success rates [34, 35]: 1) 93% in two-stage replacement with antibiotic-loaded cement, 2) 86% in two-stage replacement either without cement or using plain cement only, 3) 86% in one-stage replacement with antibiotic-loaded cement, and 4) 59% in one-stage replacement either without cement or using plain cement only.
Although two-stage prosthesis revision has been advocated as the gold standard for the treatment of infected THA [12, 36], Vielpeau C. et al. [37] reported a similar rate of infection control with either one- or two-stage replacement. In this retrospective review carried out in 14 French teaching centres, 349 fully-documented patients were followed up for at least two years after exchange arthroplasty. A cure rate of 88% was achieved for 127 patients treated by direct exchange and 85% for 222 patients treated by a two-stage procedure. They highlighted three conclusions:
- Similar success rates was observed when comparing one- and two-stage replacement (88% and 85%, respectively), as well as when comparing cementless prosthesis and those fixed with antibiotic-loaded cement (85% and 90%, respectively).
- Mechanical complications rate was higher in the two-stage replacement group. In the study, complications requiring surgery were observed in 20% of patients in the two-stage group and only 9% of the patients in the one-stage group. The complications observed were leg-length discrepancy, aseptic loosening, fractures and dislocations.
- The use of more invasive techniques was not associated with higher control of the infection. Femorotomy, which might be recommended so as to facilitate the removal of fully-coated ingrowth stems or adherent distal cement, had no influence on control of the infection. On the contrary, it was associated with a fracture rate of 14%.
More recently, a systematic review focused on reinfection rates compromising 62 relevant studies, showed that the overall rate of reinfection in patients with PJIs treated by one- or two-stage replacement was 8.6% (95% CI=4.5-13.9) and 10.2% (95% CI=7.7-12.9), respectively [38].
One-stage revision has obvious advantages in the management of infected THA, especially when performed on selected patients [39]. In fact, it has already been suggested that the overall balance of risk and benefit favours the one-stage approach over the two-stage approach for the treatment of hip prosthesis infection. The optimal treatment must balance outcome and overall risk; success requires more than simply eradicating the infection. In the case of a one-stage procedure, the patient is exposed to a single major procedure and therefore lower cumulative perioperative risk. A functional prosthesis replacement is completed without exposure to the complications associated with spacers such as spacer dislocation, femoral fractures or allergic reactions to the antibiotic [35]. In addition, there are also benefits both financially and in terms of resource allocation.
Factors associated with poor outcome are polymicrobial infection, gram-negative microorganisms, methicillin-resistent Staphilococcus aureus (MRSA) and group D Streptococcus [40]. This approach has been often underrated because of fears of recurrent infection without the use of local antibiotics delivered by cement spacers. Several factors have been associated with a successful outcome: absence of wound complications after the initial THA, good general health, sensitive Staphylococcus or Streptococcus spp. and the infecting microorganism is sensitive to the antibiotic in the cement
For some authors, the need of bone graft or cementless implants represents a contraindication for one-stage revision [2, 40]. However, others have shown good results in these situations. Winkler et al. [41, 42] published outcomes of 37 patients who were treated with one-stage cementless hip prosthesis. An infection-free rate of 92% was reported with an average follow-up of 4.4 years. In this series, five cases of MRSA were successfully treated. They conclude that allograft bone may be impregnated with high loads of antibiotics using special incubation techniques. Yoo et al. [16] published a retrospective review of 12 patients treated with a variety of cementless implants. All in all, an 83.3% implant survival was reported after a mean follow-up of 3.6 years. There was one recurrence of infection and one aseptic loosening. Rudelli et al. [43] published the outcomes of 32 patients who were treated with one-stage revision using both cemented, cementless and hybrid prosthesis. Antibiotic-
loaded cement was used with cemented prosthesis. They reported an infection-free rate of 93.7% after a mean follow-up of 103 months. García et al. [1] published a series of 14 patients with PJI treated with direct exchange, in which the femoral component was cemented in 7 cases and in 7 it was not cemented (Fig. 6). They reported no differences with regard to the clinical and microbiologic characteristics between both groups.
Surgeon training and experience should play a role in choosing the reconstruction option. Meticulous surgical debridement to clear dead space and residual bacterial colonization is emphasized by all authors. Antibiotic levels in the cement are limited as high levels can reduce mechanical integrity. Overall, it is unquestionable that a one-stage exchange, if successful, would provide the best benefit for both the patient and society. The benefits of one-stage revision make ongoing research worthwhile.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Declared none.


