All published articles of this journal are available on ScienceDirect.
A Randomized, Controlled Trial Comparing Autologous Matrix-Induced Chondrogenesis (AMIC®) to Microfracture: Analysis of 1- and 2-Year Follow-Up Data of 2 Centers
Abstract
Microfracture (MFx) is currently the recommended option for the treatment of small cartilage defects but is not regarded as suitable for the treatment of defects larger than 2.5 cm2. To extent its applicability to medium-sized defects MFx has been combined with a collagen type I/III matrix (Chondro-Gide®). This technique is called Autologous Matrix-Induced Chondrogenesis (AMIC®) and meanwhile a clinically established treatment option for localized full-thickness small- to medium-sized cartilage defects. Despite its more spreading clinical use, clinical data published so far are limited to mainly case report series.
In this study, we report the first results of a randomized, controlled trial assessing the efficacy and safety of AMIC® versus MFx. Patients enrolled in 2 centers were included in this analysis. 38 patients (aged 21-50 years, mean defect size 3.4 cm2) were randomized and treated either with MFx, with sutured AMIC® or glued AMIC®. Clinical outcomes (modified Cincinnati and ICRS score) could be assessed in 30 patients at 1-year and 27 patients at 2-years post-operation. Improvements in both scores were seen at 1-and 2-years post-operation, irrespective of the technique used. MRI assessment revealed a satisfactory and homogenous defect filling in the majority of patients. No treatment-related adverse events were reported.
This interim analysis confirms the mid-term results for AMIC® reported in literature. It demonstrates clearly that clinical outcomes at 1-year post-operation are maintained at 2-years. Therefore we consider enhancing MFx with Chondro-Gide® is a valid and safe cartilage repair option for small- to medium-sized cartilage defects of the knee.
INTRODUCTION
Cartilage defects are a frequent complication after traumatic knee injury with affected patients suffering from significant pain, morbidity and impaired mobility [1]. Due to the low intrinsic healing capacity of human articular cartilage, spontaneous healing of the damaged tissue cannot be expected [2].
Repair of the very complex articular cartilage tissue has been carried out with varying success with the goal to reduce pain, to regain mobility in affected patients by producing repair tissue that can withstand the demands of daily activity and sports, and to postpone or even avoid total knee replacement. A variety of surgical techniques that aim to resurface and repair the damaged articular cartilage have evolved. These include bone marrow stimulation methods, such as Pridie drilling, abrasion and microfracture (MFx) [3-5], implantation techniques, such as autologous chondrocyte implantation (ACI), matrix-induced autologouschondrocyte implantation (MACI) [6], and mosaicplasty [7-9]. Currently MFxis recommended as the treatment of choice for smaller cartilage defects (<2.5 cm2) [2], while ACI or MACI are frequently used for larger defects based on the publication of beneficial mid- and long-term results [7, 10-13].
Marrow stimulation methods involve penetration of the subchondral bone plate to access the bone marrow compartment [14]. The resulting blood clot enriched with bone marrow elements is thought to provide a favorable microenvironment for the development of the cartilage repair tissue. This so-called superclot is capable of further stimulating the migration, proliferation, and chondrogenic differentiation of mesenchymal stem cells from the bone marrow [14].
Autologous Matrix-Induced Chondrogenesis (AMIC®) is an innovative and clinically established treatment for localized full-thickness cartilage defects. It combines MFx treatment with Chondro-Gide® (GeistlichPharma AG, Wolhusen, Switzerland) with the goal to extent the applicability of MFx from small to medium-sized cartilage defects. Chondro-Gide®, a well-documented natural collagen type I/III matrix, is routinely used as a substitute for periost in ACI [9, 15-17] and ACI cell-seeded [18, 19] with good clinical outcomes. Its unique bilayer structure allows for optimal attachment and in-growth of cells and cartilage matrix deposition through its scaffold function and provides protection through the compact layer facing the knee cavity.
In AMIC®, the formed blood clot, which is subsequently transformed into cartilaginous repair tissue by the contained bone marrow components, is immediately stabilized by the Chondro-Gide® matrix covering the microfractured area. This may improve primary and hence secondary defect filling. An investigation of cell-laden and cell-free matrix-induced chondrogenesis techniques in a sheep model found formation of repair tissue of greater thickness when the Chondro-Gide® matrix was used [20].
The expected clinical results of AMIC® should be comparable to those reported for MFx. Generally in cartilage repair, a number of factors are critical to treatment success, including the defect filling, the integration of the tissue regenerate into the adjacent cartilage and post-operative rehabilitation program. Irrespective of the method used, clinical outcomes after cartilage repair have been reported to be better with smaller defect size and younger age, and better clinical scores are generally achieved at the femoral condyle compared with the patella [5, 11].
AMIC® is a simple, single-step procedure and may be of particular benefit when expensive two-staged implantation techniques are not an option for financial or logistic reasons.
First clinical results of AMIC® are already published, which will be discussed later in this paper [2, 21-24], but no randomized study was performed yet.
We decided to conduct an interims analysis of an ongoing, prospective, randomized, controlled trial in order to verify reported clinical findings.
MATERIAL AND METHODS
Study Design
A prospective, randomized, controlled trial (RCT) was designed to assess the efficacy and safety of the AMIC® technique compared to MFx in the treatment of small-to-medium sized cartilage defects (>2 cm2) of the knee.
Male or female between 18 and 50 years of age with 1 or 2 cartilage defects of grade III or IV according to the Outerbridge classification and a defect size between 2 and 10 cm2 were enrolled onto the study. Patients with more than 2 defects, 2 corresponding defects or defects on both knees were excluded from the study, as were those with signs of osteoarthrosis, bone lesion >0.7 cm and uncorrected knee instability. Further exclusion criteria were rheumatoid arthritis, parainfectious or infectious diseases, chronic heart, endocrine, metabolic or autoimmune disease, varus or valgus deformation, previous complete meniscus resection or mosaicplasty, treatment with cartilage specific medication (e.g. hyaluronic acid) and chondropathia patellae or dysplasia of the patella.
At arthroscopy patients were randomly assigned to 1of 3 study groups receiving the following treatments: microfracture (MFx); sutured AMIC® (fixation of Chondro-Gide® with sutures); or glued AMIC® (fixation of Chondro-Gide® with fibrin glue) by drawing a sealed envelope. The study endpoints included clinical evaluation comprising modified Cincinnati and ICRS score as well as MRI evaluation with emphasis on defect filling at 1-and 2-year follow-up. All patients agreed to undertake a strict postoperative physiotherapy program and provided informed written consent before arthroscopy was performed.
The study was approved by the Ethics Committee (03-088 and 03/173-MZ) and was conducted according to the declaration of Helsinki and Good Clinical Practice. It was not registered at a clinical trial register, because at the time of setup in 2003, such a registration was not obligatory.
The interim analysis presented here includes all patients randomized and treated at the Orthopedic Clinic, Heilig Geist Spital, Ravensburg, and the Department of Orthopedic Surgery at the University of Regensburg between January 2004 and March 2010. Not all patients were available at 1- or 2-year follow-up.
Surgical Procedures
MFx was performed according to the technique published by Steadman et al. [3, 4] as an arthroscopic procedure. For the AMIC® groups a miniarthrotomy was performed. A collagen type I/III matrix (Chondro-Gide®, Geistlich Pharma AG, Wolhusen, Switzerland) was additionally added to cover the microfractured defect area. Chondro-Gide® was placed with the porous layer facing the bone surface and fixed either using sutures (PDS 5.0, Ethicon, Norderstedt, Germany; sutured AMIC®) or by gluing the matrix onto the bone surface with fibrin glue (Tissucol, Baxter, Unterschleissheim, Germany; glued AMIC®). The stable position of the Chondro-Gide® matrix was checked by flexing and extending the knee 10 times. An intra-articular drain without suction was inserted, the wound closed, and patients were hospitalized for 2-5 days after surgery.
Postoperative Rehabilitation Program
After surgery, patients were submitted to a strict rehabilitation program. To assign all patients to the same scheme was judged appropriate since AMIC® as enhanced MFx is based on bone marrow stimulation and involves the same cartilage healing mechanism as MFx alone.
The staged program included increasing weight bearing and mobilization exercises, electrotherapy of leg muscles, proprioception, walking, and sports as indicated in Table 1.
Proposed Postoperative Treatment Scheme
| 0-10 Days | Up to 3 Weeks | 3-6 Weeks | 7-12 Weeks | 4-6 Months | From 7 Months | |
|---|---|---|---|---|---|---|
| Pain treatment | Analgesia Ice application | Analgesia Lymph drainage Muscle stimulation Thermotherapy | Lymph drainage Electrotherapy Muscle stimulation | Electrotherapy Muscle stimulation | ||
| Weight bearing | Foot sole contact 3 point walk with crutches | Foot sole contact 3 point walk with crutches | Foot sole contact Walking with crutches | Building up full weight bearing Full weight bearing from 8th week | Full weight bearing | |
| Mobilization | Orthesis: 0/0/60° femoral condyle, 0/0/30° patella and trochlea Patella mobilization in all directions | Active motion in pain-free region Patella mobilization in all directions Orthesis/CPM*/CAM*: 0/0/90° femoral condyle and patella and trochlea from week 4 Increase time interval | Active motion Patella mobilization in all directions Weaning off orthesis Orthesis/CPM*/CAM*: 0/0/90° femoral condyle, patella and trochlea from week 4 Increase time interval | Active motion 0/0/90° for all defect locations from 6th week Full flexion allowed when full weight bearing achieved | Patella guidance 0/0/120° | Patella guidance Release motion |
| Electrotherapy | Quadriceps Isometrics Muscle stimulation | Quadriceps isometrics Movement of leg in extension Muscle stimulation | Leg press | Leg press | Leg press | |
| Proprioception | Manual therapy of foot joints Rubbing/brushing sole of foot PNF pattern with other leg | Foot resting on ball Different foot sole contact while sitting in many different knee positions | PNF* pattern with operated leg with knee extended Foot resting on ball | PNF* pattern with operated leg including knee flexion Trampoline Soft floor mats Equilibrium training | PNF* pattern with operated leg including knee flexion Trampoline Soft floor mats Equilibrium training | |
| Walking | Mobilization 3 point walk | Walking in water Walking stairs with crutches | Walking in front of mirror | Weaning off crutches Stepper Walking long distances on different ground Increase velocity | Walking on soft ground | Jogging |
| Sport | Aquatraining | Aquatraining Swimming Ergometry | Aquajogging Biking | Swimming Biking Ergometry 0/0/120° | Jogging, skiing after 9-12 months Skating after 12 months Biking Contact sport after 18 months |
* CPM: Continuous passive motion; CAM: Continuous active motion; PNF: Proprioceptive neuromuscular facilitation.
Scar tissue management for the patients with mini-arthrotomy is not specifically listed in this table but was part of the clinical routine.
Assessment of Clinical Outcome and Safety
Clinical outcome was assessed by physical examination, and using the Modified Cincinnati [15] and Modified International Cartilage Repair Society (ICRS)[25] Scores. The Modified Cincinnati Score is divided into the three parts: assessment of knee function (6-30 points); clinical pathology (0-20 points); and highest activity level without pain (0-50 points) [15]. A maximal score of 100 points is possible. The Modified ICRS Score consists of ratings carried out by the patient and the surgeon. Patients were asked to rate their pain and the functional status of the affected knee. In this study, pain was assessed using a visual analogue scale from 0 (no pain) to 100 (severe pain). Functional status was rated according to the ICRS Cartilage Injury Standard Evaluation Form-2000 from normal to severely abnormal. The affected knee was evaluated by the surgeon with respect to functional status (i.e. knee-related limitation in the daily activity), classification, and crepitation, utilizing parts 3, 4 and 7 of the ICRS form. Classification of the affected knee was performed using the Lachman test, valgus and varus rotation, and pivot shift. Each test was graded from normal to severely abnormal, and the lowest grade within an assessment determined the final grade for this assessment.
Safety was evaluated by physical examination and continuous monitoring of adverse events.
MRI Analysis
Structural regeneration of the cartilage defect was assessed by magnetic resonance imaging (MRI) performed by an independent radiologist, with a focus on the extent, signal intensity and surface of the defect filling, integration to adjacent cartilage, and bone marrow lesion (BML).
This adapted scoring system takes into account a variety of features that are currently believed to be relevant to the integrity of cartilage repair tissue as used in the MOCART-score [26] after ACI and semiquantitative MRI-scores of osteoarthritis established as WORMS [27] and BLOKS [28].
Statistical Analysis
Inferential data analysis of treatment effects was performed by the Brunner-Langer-approach [29], suitable for analyzing designs of this type. By its non-parametric construction, it is especially suitable for small samples. Since Brunner-Langer-models are able to deal with incomplete records (under the general assumption of missing completely at random), all available data (not only those from patients with complete records) were used in this analysis.
Due to the exploratory nature of this interim analysis, no correction for multiple testing is performed; thus the significance level of 5% is used as a threshold for hypotheses testing. For each of the endpoints listed in the next section, two questions were addressed:
- Do the values differ systematically over time? (e.g. main effect of time).
- Do the changes over time differ systematically between treatments? (e.g. interaction effect between treatment and time).
All statistical analyses were performed using the statistics software R version 1.13.0. [30].
RESULTS
Baseline Characteristics
38 patients aged between 21 and 50 years with isolated cartilage defects of Grade III-IV according to the Outerbridge Classification were randomized and treated. From those, 10 were treated with MFx, 13 with sutured AMIC®, and 15 with the glued AMIC® technique. Seven patients (2 in the MFx, 4 in the sutured AMIC® and 1 in the glued AMIC® group) could not be evaluated at either 1-or 2-years post-operation and for 1 patient in the glued AMIC® group only 2-year results were available. MRI data were missing for 1 patient at 1-year post-operation in the sutured AMIC® group and for 2 patients at 2-years in the MFx goup. Assessment of clinical outcome and safety at 1-year post-operation was therefore performed in 30 patients (8 in the MFx group, 9 in the sutured AMIC® and 13 in the glued AMIC® group). For 27 patients (6 in the MFx group, 8 in the sutured AMIC® group, and 13 in the glued AMIC® group) 2-year results were available at the time of the interim analysis (Fig. 1).
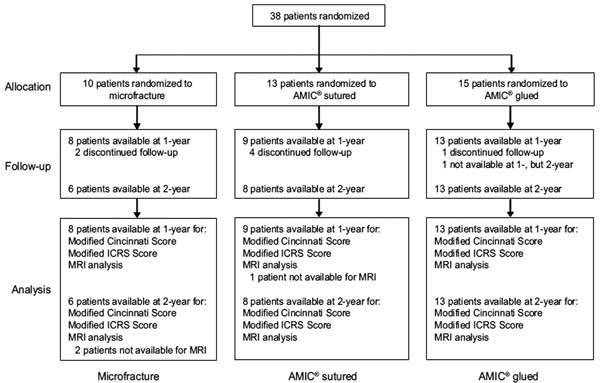
Enrollment and outcome measures for the 3 treatment options: microfracture, sutured AMIC® and glued AMIC®.
Characteristics of the patients included in the interims analysis are shown in Table 2.
Patient Characteristics at Surgery
| Patients Data at Surgery | Patients with Available 1-Year Data | Patients with Available 2-Year Data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MFx | Sutured AMIC® | Glued AMIC® | MFx | Sutured AMIC® | Glued AMIC® | MFx | Sutured AMIC® | Glued AMIC® | |
| (n=10) | (n=13) | (n=15) | (n=8) | (n=9) | (n=13) | (n=6) | (n=8) | (n=13) | |
| Age, mean (SD) | 41 (5) | 33 (8) | 38 (8) | 42 (6) | 34 (9) | 39 (8) | 41 (6) | 35 (8) | 39 (8) |
| Male sex, n (%) | 8 (80) | 11 (85) | 12 (80) | 6 (75) | 7 (78) | 10 (77) | 4 (67) | 7 (88) | 10 (77) |
| Height (cm), mean (SD) | 178 (9) | 177 (9) | 177 (7) | 177 (8) | 175 (8) | 177 (8) | 176 (9) | 176 (9) | 177 (8) |
| Weight (kg), mean (SD) | 78 (10) | 87 (15) | 86 (10) | 78 (9) | 82 (13) | 86 (10) | 79 (10) | 84 (12) | 86 (10) |
| BMI, mean (SD) | 24.6 (1.9) | 27.8 (4.8) | 27.7 (3.7) | 24.9 (1.8) | 26.6 (4.8) | 27.7 (3.9) | 25.2 (2.0) | 27.4 (4.6) | 27.7 (3.9) |
| Defect class, III:IV, n | 4:6 | 7:6 | 6:9 | 2:6 | 4:5 | 6:7 | 1:5 | 3:5 | 5:8 |
| Defect size (cm2), mean (SD) | 2.9 (0.7) | 3.7 (1.1) | 3.5 (1.1) | 3.1 (0.7) | 3.7 (1.2) | 3.7 (1.2) | 3.1 (0.8) | 3.8 (1.2) | 3.8 (1.2) |
| Patients with prior operation, n (%) | 5 (50) | 8 (62) | 8 (53) | 5 (63) | 5 (56) | 6 (46) | 3 (50) | 5 (63) | 6 (46) |
| Patients with meniscus revision, n (%) | 6 (60) | 4 (31) | 4 (27) | 6 (75) | 3 (33) | 4 (31) | 4 (67) | 3 (38) | 4 (31) |
| Patients with injury, n (%) | 2 (20) | 5 (54) | 7 (33) | 2 (25) | 4 (44) | 5 (38) | 2 (33) | 4 (50) | 4 (31) |
Mean age at surgery was 37 years (range 21—50). 21 patients had previously undergone surgery at the affected knee and the mean defect size after debridement was 3.4 cm2 (range 2.1—6.6 cm2). Baseline characteristics were largely comparable between groups. The proportion of patients with meniscus revisions was higher and the proportion of patients with defect class III was lower in the MFx group compared with the other 2 groups. The proportion of patients with injuries was higher in the sutured AMIC® group compared with the other 2 groups for patients with available 2-year data, whereas it was lower in the MFx group compared with the other 2 groups for patients with available 1-year data.
Statistical analysis revealed no significant changes also due to the small patient number in the different treatment groups. Therefore descriptive statistics has been chosen for this report.
Clinical Outcomes at 1- and 2-Years
24 patients showed an improvement in the Modified Cincinnati Score at 1-year post-operation. Mean scores increased significantly from baseline values of 37 ± 14 for the MFx group (including one patient who presented with a poor Cincinnati Score of 7 at screening), 47 ± 20 for the sutured AMIC® group and 47.0 ± 15 for the glued AMIC® group to 68 ± 17 (p = 0.002), 82 ± 14 (p< 0.001) and 67 ± 27 (p = 0.02), respectively, at 1-year post-operation. The mean change from baseline to 1-year was slightly higher in the MFx and sutured AMIC® groups (31 ± 13 and 35 ± 29, respectively) compared with the glued AMIC® group (19 ± 22), but there were no statistical significances between the groups (Fig. 2).
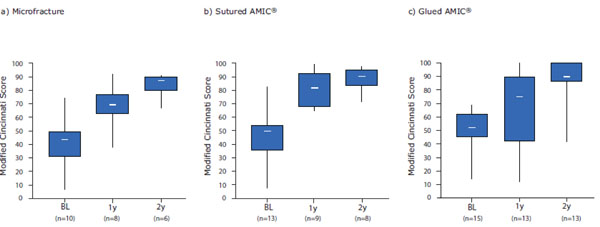
Box and Whisker plot of the Modified Cincinnati Scores at baseline (BL) and at 1-(1y) and 2-years (2y) post-operation for the three treatment groups: MFx (a), sutured AMIC® (b) and glued AMIC® (c). Scores are presented as medians; the ends of the boxes define the 25th and 75th centiles and the minimum and maximum score is indicated.
At 2-years post-operation mean scores increased significantly from baseline values of 40 ± 9 for the MFx group, 43 ± 16 for the sutured AMIC® group and 48 ± 15 for the glued AMIC® group to 83 ± 8 (p< 0.001), 88 ± 9 (p< 0.001) and 85 ± 18 (p< 0.001), respectively (Fig. 2). The mean change from baseline to 2-years post-operation was comparable between the groups without statistical significances (44 ± 15 for MFx, 46 ± 17 for sutured AMIC® and 37 ± 14 for the glued AMIC® group). 12 patients (3 in the MFx, 3 in the sutured AMIC® and 6 in the glued AMIC® group) showed a further improvement of more than 10 points in the Modified Cincinnati Score during the second year. 12 patients largely maintained the score from 1-year post-operation, while 2 patients in the sutured AMIC® group and 1 patient in the glued AMIC® group showed a slight deterioration in the score. In the glued AMIC® group, 1 patient did not present at the 1-year follow-up assessment, but presented with an improved score at 2-years post-operation compared with baseline. Interestingly, 3 patients in the glued AMIC® group who had presented with an inferior score at 1-year post-operation compared with baseline, showed a clear improvement during the second year to above baseline levels.
With regards to the Modified ICRS score, 24 patients rated their functional status as improved at 1-year post-operation, while 6 patients rated their functional status as stable (Fig. 3). At 2-years post-operation 12 patients improved their functional status between 1-and 2-years, 13 remained stable and 2 patients deteriorated (sutured AMIC®) from normal to nearly normal.
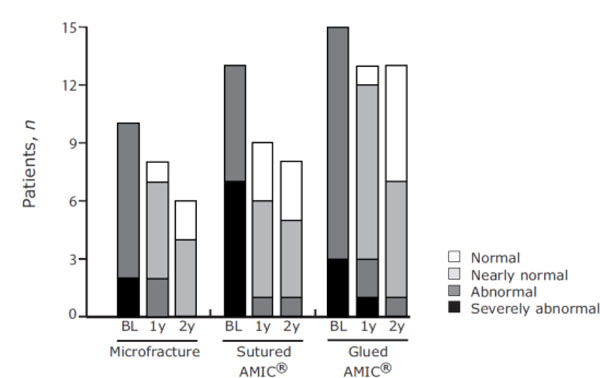
Modified ICRS Score - patient assessments. Functional status was rated by the patient at baseline (BL) and at 1- (1y) and 2-years (2y) post-operation for the three treatment groups. Stacked bar charts show the number of patients with the corresponding functional status for each treatment group at BL and at 1 and 2 years.
Pain was rated as less severe at 1- and 2-years post-operation compared with baseline (pre-operative) and was comparable between the groups. Baseline pain was 54 ± 21 for MFx, 46 ± 19 for sutured AMIC® and 48 ± 20 for glued AMIC®. At 1-year post-operation pain decreased significantly to 19 ± 17 for MFx (p = 0.002), 14 ± 13 (p< 0.001) for sutured AMIC® and 16 ± 13 (p< 0.001) for glued AMIC® and was further reduced at 2-year post-operation without statistical significance (5 ± 3 for MFx, 9 ± 6 for sutured AMIC® and 10 ± 13 for glued AMIC® (Fig. 4).
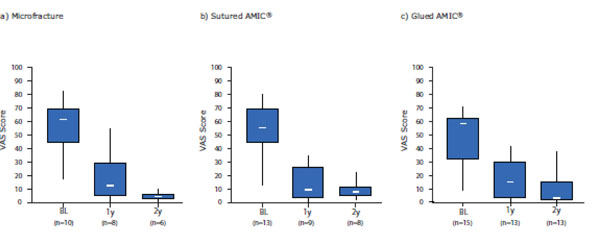
Box and Whisker plot of the Modified ICRS Score - patient assessments. Pain was rated by the patient at baseline (BL) and at 1- (1y) and 2-years (2y) post-operation for the 3 treatment groups. Scores are presented as medians; the ends of the boxes define the 25th and 75th centiles and the minimum and maximum score is indicated.
In the sutured AMIC® group, 1 patient presented with low pain at baseline had a slightly increased pain Score at 1-year post-operation, but improved thereafter back to baseline and 1 patient in the glued AMIC® group rated his pain increasing over time starting with very low pain at baseline and light pain at 2-years.
In addition to the patient-rated assessments, three Modified ICRS Score assessments were performed by the surgeon. These included classification of the affected knee, crepitation and functional status and no statistical significances were found between the groups (Fig. 5).
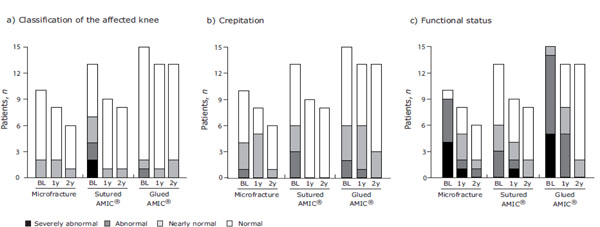
Modified ICRS Score - surgeon assessments. Classification of the affected knee (a), crepitation (b), and functional status (c) were assessed by the baseline (BL) and at 1- (1y) and 2-years (2y) post-operation for the three treatment groups. Stacked bar charts show the number of patients with the corresponding functional status for each treatment group at BL and at 1 and 2 years.
With regards to classification of the affected knee, 6 patients improved and 24 patients remained stable 1-year post-operation. At 2-years post-operation 1 patient (MFx) improved between 1- and 2-years, 25 remained stable and 1 patient (glued AMIC®) deteriorated from normal to nearly normal.
The crepitation score improved in 10 patients and remained stable in 15 patients at 1-year post-operation, while 5 patients slightly deteriorated. At 2-years post-operation 6 patients improved their score between 1- and 2-years and 21 patients remained stable.
The functional status as rated by the surgeon was improved after 1-year in 25 patients, remained stable in 3 patients and 2 patients deteriorated from abnormal to severely abnormal. At 2-years post-operation 8 patients further improved their functional status between 1-and 2-years as well as the 2 patients with a deterioration after 1-year. 16 patients remained stable and 1 patient deteriorated (MFx) from nearly normal to abnormal.
MRI Evaluation
Assessment of the degree of defect filling and the surface and integration of the regenerate was performed by MRI at 1- and 2-years post-operation. One-year results were available for 29 patients, 2-year results for 25 patients (Table 3).
MRI Evaluation at 1- and 2-Years
| MFx | Sutured AMIC® | Glued AMIC® | |||||
|---|---|---|---|---|---|---|---|
| 1 Year | 2 Years | 1 Year | 2 Years | 1 Year | 2 Years | ||
| (n=8) | (n=4) | (n=8) | (n=8) | (n=13) | (n=13) | ||
| Defect filling | none | 0 | 0 | 0 | 0 | 1 | 1 |
| 1/3 | 2 | 0 | 1 | 1 | 0 | 3 | |
| 1/3-2/3 | 2 | 1 | 4 | 2 | 4 | 1 | |
| > 2/3 | 4 | 3 | 3 | 5 | 7 | 8 | |
| not evaluable | 0 | 0 | 0 | 0 | 1 | 0 | |
| Surface | largely uneven | 2 | 1 | 3 | 2 | 3 | 2 |
| partially uneven | 4 | 0 | 4 | 4 | 9 | 5 | |
| smooth | 2 | 3 | 1 | 2 | 0 | 5 | |
| not evaluable | 0 | 0 | 0 | 0 | 1 | 1 | |
| Integration | marginal gap up to 50% | 0 | 1 | 2 | 0 | 0 | 0 |
| marginal gap | 7 | 0 | 3 | 3 | 9 | 8 | |
| complete | 0 | 3 | 2 | 5 | 3 | 3 | |
| not evaluable | 1 | 0 | 1 | 0 | 1 | 2 | |
| Signal intensity of defect cover | inhomogeneous | 5 | 0 | 5 | 4 | 6 | 4 |
| homogenous | 3 | 4 | 3 | 4 | 6 | 8 | |
| not evaluable | 0 | 0 | 0 | 0 | 1 | 1 | |
| Bone marrow lesion | massive (> 2cm) | 0 | 1 | 0 | 0 | 1 | 1 |
| intermediate (1-2 cm) | 4 | 0 | 2 | 2 | 4 | 5 | |
| small (< 1cm) | 3 | 1 | 6 | 4 | 6 | 5 | |
| none | 0 | 2 | 0 | 2 | 2 | 3 | |
| not evaluable | 1 | 0 | 0 | 0 | 0 | 0 | |
At 1-year post-operation, 14 patients showed a defect filling of two thirds or more. Complete integration was observed in no patient in the MFx group, 2 patients in the sutured AMIC® group and 3 patients in the glued AMIC® group. The quality of the regenerate surface and defect cover were similar in all 3 groups; with a trend towards reduced surface quality, but better defect cover in the glued AMIC® group. The occurrence of bone marrow lesions was comparable between the groups and no further ossification has been observed.
Fig. (6) shows an example of an MRI evaluation performed 12 months post-operation in 1 patient treated with glued AMIC®, demonstrating a complete and almost homogenous filling of the defect.
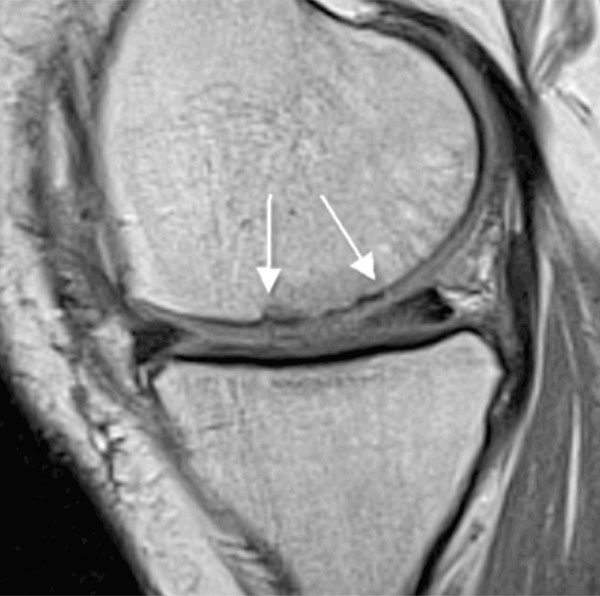
MRI follow up at 1 year post-operation (glued AMIC®, medial femoral condyle): Proton density weighted sequence (PDw) in sagittal orientation. Defect filling almost complete (20 x 20 mm, see arrow), surface remaining slightly uneven, good marginal integration, cartilage repair tissue isointense to adjacent genuine cartilage.
At 2-years post-operation, defect filling was largely comparable between the groups without statistical significances. Complete integration or with marginal gap was seen in 3 patients in the MFx group, 8 patients in the sutured AMIC® group and 11 patients in the glued AMIC® group.
Safety
No treatment-related adverse events were reported for any of the patients included in this interim analysis. One patient treated with glued AMIC® received a joint replacement after 1-year and dropped out of the efficacy analysis.
DISCUSSION
AMIC® combines MFx treatment with the application of Chondro-Gide® and was developed with the aim of offering a simple and effective treatment for small- to medium-sized cartilage defects (>2 cm2). To gather information on the medium-term effectiveness of AMIC® a RCT in patients with cartilage defects of the knee was started in 2004.
It was originally planned to include 120 patients, 40 in each group. Accordingly computer assisted block randomization was carried out in blocks of 30. Since the 2 sites included in this interim analysis could not enroll all their allocated patients yet, the number of patients in each study group varies and is particularly low in the MFx group. This makes comparisons between the groups even more difficult.
Already at the beginning of the study it proved to be extremely difficult to convince patients to participate, since they did not want to be treated with MFx only. Hence, recruitment was very slow and is still ongoing to enroll the adequate number of patients. It is even more difficult as AMIC® is a clinically established cartilage repair option, available without participation in a study.
Discussion and justification of the study design is necessary for several reasons. When AMIC® was developed, Chondro-Gide® was thought to be sutured to the defect site, as in ACI. Since fixation with fibrin glue had been established for MACI, a third group - glued AMIC®, was added. Meanwhile gluing is routinely carried out and proved to be a feasible fixation technique. It has been shown that surgical suturing of articular cartilage can induce osteoarthritis-like changes [31]. Therefore, the sutured AMIC® technique has largely been replaced by glued AMIC®. The superiority of gluing is supported by published results demonstrating that biphasic carrier constructs, consisting of fibrin glue and a Chondro-Gide® matrix, improve chondrogenesis [32].
In 2003 when the study was designed different outcome scores for cartilage were used. Here the Cincinnati, not specific for cartilage as for instance the Knee injury and Osteoarthritis Outcome Score (KOOS), was chosen in addition to the ICRS score.
The postoperative rehabilitation protocol was the same for all treatment groups since AMIC® as enhanced MFx is based on bone marrow stimulation and involves the same cartilage healing mechanism as MFx alone. A possible effect of the different surgical approach, arthroscopic versus mini-arthrotomy, was not addressed in this study. The arthroscopical approach is less invasive compared to mini-arthrotomy, but effects on cartilage healing are unlikely to be still detectable at 1-year. No adverse events, e.g. pain or impaired motion related to scarring were reported.
Overall, our data are in agreement with the results of Gille et al. reporting a significant improvement in clinical outcomes in patients treated with AMIC®, assessed by 5 different scores up to 36 months. 87% of their patients treated with AMIC® were subjectively highly satisfied with the results after surgery [22]. Although patient satisfaction with the treatment chosen was not directly assessed in our analysis, the positive results obtained not only in scores assessed by the surgeon, but also in subjective patient ratings are encouraging.
The findings presented here demonstrate improved Modified Cincinnati and ICRS scores and an improvement in clinical symptoms at 1- and 2-years post-operation for most of the patients, irrespective of the technique used.
Mean changes from baseline in the Modified Cincinnati Scores were slightly higher in the MFx and sutured AMIC® groups compared with the glued AMIC® group at 1-year, but comparable at 2-years post-operation. Interestingly, the glued AMIC® group comprised 1 patient who presented with a worsened Cincinnati Score at 1-year post-operation, but who showed clinical improvement to above baseline scores at 2-years. In agreement with results published by Gille et al. [22], where further improvements in Cincinnati scores were observed up to 24 months post-operation, this may indicate that the beneficial effects of AMIC® may become more prominent after the first post-operative year. While the majority of patients either remained stable or showed further improvement during the second year post-operation, 1 patient in the glued AMIC® group presented with decreased Cincinnati Scores at 2-years post-operation, despite having shown initial improvement at the 1-year assessment.
The ICRS score largely remained stable or further improved at 2-years compared to 1-year post-operation. This result is again in agreement with published results of AMIC® where a significant increase in mean ICRS values was noted at 12 and 24 months post-operation [22].
In this study, pain was found reduced in all treatment groups at 1-year post-operation and further decreased at 2-years in the majority of patients. Only 1 patient in the glued AMIC® group reported increased pain over time; however, pain was rated very low at baseline and as light pain at 2-years post-operation.
When drawing conclusions from the clinical evaluations, especially assessments conducted by the patients themselves, it needs to be taken into account that these are subjective and may, for example, depend on patient expectations. Here differences in patient rated assessments and surgeons rated ones were not analyzed since sample sizes were too small to draw conclusions. However, in the ICRS score patients rated their part of functional status overall lower than the surgeons by clinical assessment. Both reported a positive trend over time.
MRI assessment revealed good defect filling in the majority of patients, independent of the treatment method. The proportion of patients with homogenous repair tissue was found to be approximately 50% at 1-year post-operation in all 3 groups. It remained 50% in both AMIC® groups at 2-years but improved in the 4 MFx patients with available 2-year MRI data to 100% homogenous. Radiologic evaluation showed slightly inferior outcome in the AMIC® groups, especially with regard to the surface of the regenerate and degree of integration. However, in the 2-year analysis of clinical outcomes, which included 4 patients treated with MFx, these observations were not confirmed; outcomes were satisfying and comparable for all groups. Indicators for poor mid- and long-term outcome could be a possible thickening of the subchondral layer, bony overgrowth, or the formation of subchondral cysts after bone marrow stimulation and were not observed yet in the study population [33-36].
Studies have suggested that the repair tissue formed after ACI may be more hyaline-like and therefore of higher quality and durability than the regenerate formed following bone marrow stimulating techniques, such as MFx [22, 37, 38]. However, the association between hyaline-like structural repair and clinical outcome remains controversial [37, 39, 40]. In one comparative study, ACI and MFx provided similar clinical and radiographic results 2 and 5 years after surgery, and no correlation between histological findings and clinical outcome could be shown [41].
Comparison of these results with medium-term results of other cartilage regeneration techniques is difficult due to the small analysis population in this assessment, and because of the different evaluation and scoring systems used. To date, no randomized controlled studies have been published comparing AMIC® with other cartilage repair procedures. Several randomized studies have, however, compared outcomes after ACI, MFx or transplantation techniques [9, 39, 41-45]. Whilst ACI was reported to be superior to mosaicplasty in one study [9], other authors found these techniques to be clinically equivalent [39]. In studies comparing ACI and MFx, both techniques provided satisfactory outcomes at 2- and 5-years after surgery, with no significant differences in the clinical and radiographic results between the 2 treatments [41, 43]. Evaluation of clinical and histological outcomes after ACI and osteochondral cylinder transplantation revealed a decrease in symptoms with both techniques, but the onset of recovery was slower with ACI [42]. Characterized chondrocyte implantation (CCI) was found to produce superior structural repair to MFx 1-year after treatment. However, clinical outcomes in the KOOS were similar between both treatment groups at 1-year post-operation [44]. After 3 years, the mean improvement in overall KOOS from baseline was greater for the CCI group. A positive treatment effect was however not seen in all subdomains of the score [33]. Conflicting data have also been published regarding the durability of different cartilage repair strategies. Whilst some groups have reported mid- and long-term survival rates of approximately 75% after MFx [5, 41], others have observed that good short-term results are not maintained in the long-term [35]. Despite these controversies, failure rates after MFx were found to be about 25-28% [35], which is comparable to values for alternative biologic treatment options, such as ACI [41].
In this analysis, the small study population with available 1- and/or 2-year data allowed only a limited statistical comparison between the treatment groups, and due to the limited follow-up of 2 years, conclusions with regards to differences in clinical outcomes and the quality of the repair tissue cannot be drawn. However, as stated before, AMIC® is aimed to extent the applicability of MFx from small- to medium-size cartilage defects and results are expected to be equivalent to MFx.
Unfortunately, 8 patients were lost to follow-up despite that they had been informed and agreed about the mandatory 1- and 2-year visits. However, except for 2 patients, which came back at 2-years and 5-years respectively, all others presented at least at 6-months post-operation. 1 patient had to undergo total knee arthroplasty then. He had been treated before AMIC® 4 times with lavage and debridement for persistent pain and had a relatively high body mass index (BMI) of 29. It is well known that the number of previous surgeries and BMI influences clinical outcome after cartilage repair [29]. The remaining 5 patients either moved away without leaving their new address or did not conclude their study follow-up without giving a reason. This is a well-known and often experienced issue which is very rarely described or discussed, especially in medical device industry. Non-compliance might be attributed to following reasons: either the patient is satisfied with treatment outcome and feels no need for further participation or he is not satisfied and refuses continuation.
Due to the reasons outlined above, definite conclusions about the mid- to long-term effectiveness of AMIC® are currently not possible. However, this study confirms the effectiveness and safety of AMIC® glued or sutured and demonstrates that the good results observed at 1-year post-operation are maintained at 2-years. Pain reduction and improvement of symptoms and hence function could be achieved up to 2-years post-operation. Five-year results are expected and may provide important insights into the long-term effectiveness of AMIC® for the treatment of cartilage defects of the knee.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
Medical Writing Support was provided by nspm ltd, Meggen, Switzerland. The authors would like to thank Holger Klöss and Katja Martin (GeistlichPharma AG, Switzerland) for their continued support during the study, the preparation of the manuscript and their critical comments throughout the review process.


