All published articles of this journal are available on ScienceDirect.
Expressional Analysis of GFP-Tagged Cells in an In Vivo Mouse Model of Giant Cell Tumor of Bone
Abstract
Giant cell tumor of bone in a neoplastic stromal cell which survives for multiple passages in primary cell culture with a stable phenotype. In the pathological environment of GCT, the neoplastic nature of the mesenchymal stromal component drives local hematopoietic precursors to undergo fusion and form multinucleated osteoclast like giant cells. There is currently very limited knowledge about the pathogenesis of GCT due to the lack of suitable in vivo models for this tumor. Here we report stable gene transfer of Green fluorescence protein (GFP) in GCT stromal cells. In the present study, we have used GCT stromal cells that stably express enhanced green fluorescence protein (GFP) that are used in a new in vivo culture model. Our results show the utility of the GFP tagged cell lines that stably express GFP signals up to 52 weeks of continuous growth. The in vivo model described herein can serve as an excellent system for in vivo therapeutic and mechanistic evaluation of existing and novel targets for GCT.
INTRODUCTION
Giant cell tumor of bone (GCT) is an aggressive tumor of bone characterized by presence of multinucleated giant cells which occasionally metastasis to lungs. It typically affects the epiphyseal regions of the long bones such as the distal femur, the proximal tibia, and the distal radius, prompting the formation of a local osteolytic lesion [1-7]. There is currently no effective curative treatment for GCT other than aggressive surgical resection [8-11].
Metastasis, with identical morphology to the primary tumor, occurs in a few percent of cases, usually to the lung [12-14]. There is a need for clinically relevant in vivo models to study the molecular and cellular mechanisms underlying metastatic behavior. The major obstacle to the treatment of GCT is the lack of effective pre-and post-operative adjuvant therapy and the lack of an animal model that accurately reflects the pathophysiology of human GCT.
The aim of this study was to develop a clinically relevant in vivo model using exogenous gene transfer of GFP into the GCT stromal cell which would allow assessment of in vivo response to systemic treatment. Here we report a successfully generated GCT stable cell line and GFP expression in an in vivo mouse model of GCT. This current in vivo mouse model will be beneficial for the clinical prospective to characterize the systemic therapeutic targets in GCT and to understand the pathobiology of GCT.
MATERIAL AND METHODS
Primary Cell Line Culture and Transfection
GCT cell culture and transfection assay were performed as described in our previous study [15]. Briefly, GCT stromal primary cells were obtained from fresh tissue of GCT patient following Ethics Board approval and patient consent. The GCT tissue was processed and cell suspension was cultured in DMEM containing glutamine and supplemented 10% fetal bovine serum, 1% penicillin/streptomycin. After several successive passages, the multinucleated giant cells were eliminated from the culture and only mesenchymal stromal cells remains in the culture. Cells transfection was carried out in e-GFP vector using electroporation method [15].
PCR Detection
Total RNA extraction was performed by TRIZOL Reagent (Sigma) according to the manufacturer’s protocol. The resuspended RNA samples were treated with ribonuclease A (RNase A)-free DNaseI for 1h at 37°C to remove residual genomic DNA. One microgram of total RNA was incubated with 2 ml primer cocktail at 68ºC and subjected to reverse transcription (RT) using Superscript III reverse transcriptase (Invitrogen) for cDNA synthesis. An aliquot of 5 μl of the reverse transcription reaction was used in a 50-μl PCR reaction. GFP signals were detected by PCR analysis using GFP (F). AAGTTCATCTGCACCACCG and GFP (R) TCCTTGAAGAAGATGGTGCG primers, respectively. The PCR conditions were 94°C denaturation for 3 min followed by 40 cycles of 94°C for 30 sec, 55°C for 40 sec, and 72°C for 50 sec. The RT-PCR products were separated on a 2% agarose gel. A 300-bp band represented the eGFP mRNA, and a 250-bp band indicated β-actin as internal control.
Protein Extraction and Western Blot
Cytoplasmic fractions were isolated from GFP-transfected cells by scraping after 24 h of incubation and then centrifuged for 5 min. The cells were lysed with NP-40 containing lysis buffer (10 mM Tris, pH 7.4, 10 mM NaCl, 5mM MgCl2, 0.5% NP-40) to disrupt the cell membrane and then the cell lysate was centrifuged at 500 × g for 5 min at 4°C. The supernatant (cytoplasmic fraction) was removed. Proteins were denatured by boiling in sample buffer, separated on 12% SDSPAGE and then transferred onto the PVDF membrane (Immobilon TM-PSQ, Millipore) and blocked overnight in 5% non-fat powdered milk in TBST (10 mM Tris-HCl pH7.5,100 mM NaCl, 0.1% (v/v) tween-20). Mouse monoclonal anti-GFP antibody (1:1000 diluted in TBST) (Abcam) was used for protein detection. Peroxidase conjugated goat anti-mouse IgG (1:5,000 diluted in TBST) (Sigma, Missouri, USA) was used as a secondary antibody.
In Vivo GFP Expression
Suspensions were made of one of the GFP-transfected GCT cells prior to injection in phosphate-buffered saline. Injections were prepared using 1cc syringes fitted with 27-ga needle and drawing up 0.01 mL of injectate, corresponding to a total of 105-106 cells per injection. 6-7 week old female Balb/c nu/nu mice were anaesthetized using gaseous isoflurane and injected into the sub-periosteum of the right tibia. The left tibia was injected with PBS to act as an internal negative control. Mice were monitored weekly for the presence of tumors, and then 2-3 times weekly when tumor growth was noticed. The mice were sacrificed at day 8 and 35 post-injection and stored in formalin. Both lower limbs and any tumours were then removed for further analysis. Both limbs were decalcified for two weeks (TBD-2 Decalcifier, Thermo Scientific) and processed (Shandon Citadel 2000 Tissue Processor). Decalcified limbs were embedded in paraffin wax, cross-sectioned and mounted to microscope slides.
Immunofluorescence Assays
Immunofluoresence assays were conducted according to standard protocols used by singh et al. 2010. Briefly, culture chamber slides containing the GFP-transfected GCT cells as well as slides of the sectioned tissues were first fixed in paraformaldehyde and the cells were permeabilized with Triton X-100. After blocking slides with BSA, these slides were incubated for 1h at 37°C with the rabbit polyclonal anti-GFP primary antibody (1:250 dilutions; Abcam, Cambridge, MA). Slides were further incubated in secondary antibody Texas Red conjugated goat anti-rabbit IgG (1:1000 dilutions; Invitrogen) for 1h at room temperature. The slides were washed and incubated with DAPI for three minutes and then mounted using glycerol.
RESULTS
Transfection Efficiency of eGFP in GCT Stromal Cells
GCT stromal cells were transfected with eGFP-C1 construct using an electroporation method. To determine the cellular localization of green fluorescence protein, transfected cells were counted under fluorescence microscopy. GFP expressions were detected in successfully transfected GCT stromal cells cytoplasm and in the nucleus (Fig. 1a). The expression of GFP in GCT stromal cells was validated by semi-quantitative PCR using GFP specific primers and Western blot analysis. We observed GFP expression in transfected cells, but not in untransfected GCT stromal cells by semi-quatitative PCR (Fig. 1b) and confirmed GFP expression in Western blot analysis using an anti-GFP antibody (Fig. 1c).
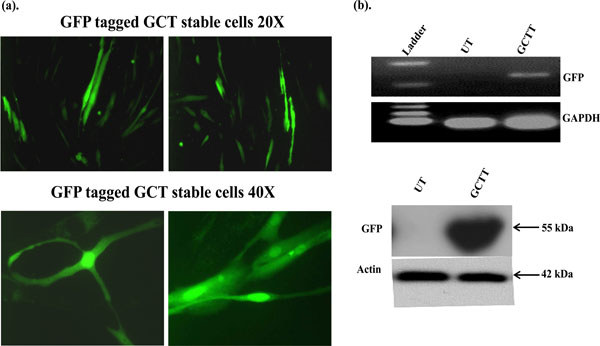
Subcellular localization of GFP signals in GCT transfected stromal cells. GFP signals were detected by immune-fluorescence. (b) Semi-quantitative PCR: GFP expression in transfected GCT stromal cells was detected using GFP specific primers and GAPDH was used as an internal control. UT=untrasnfected cells and GCTT= GCT transfected cells. (c) These results are verified at the protein level by western blot analysis.
Generation of Stable GCT Stromal Cells
A highly invasive and spontaneously metastatic GCT human cell line was transfected with the enhanced green fluorescent protein (GFP) as previously described and treated with cell media containing 1µg/ml G418 (Invitrogen). Positive GFP transfected cells were selected under fluorescence microscope, transferred and subcultured with G418 media. Stable expression of GFP in GCT stromal cells was confirmed in cells cultured in vitro up to 52 weeks of continuous growth. These results show the in vitro utility of the GCT stromal cell lines that stably express high levels of GFP (Fig. 2).
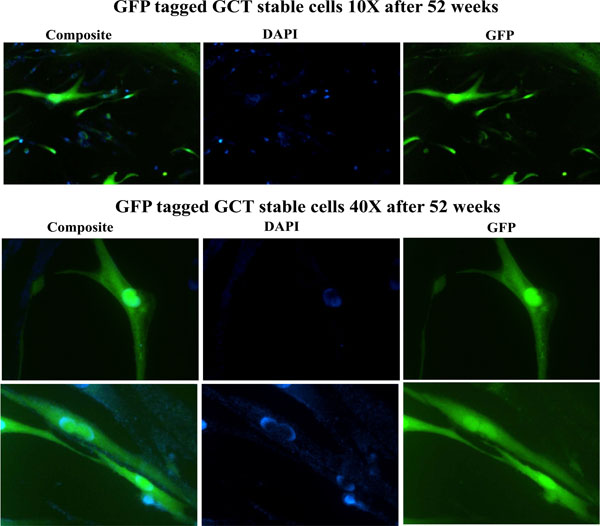
Detection of GFP signals after 52 weeks. Stable expression of GFP was confirmed in cells cultured in vitro up to 52 weeks of continuous growth. GFP expression was detected under fluorescence microscopy. DAPI (blue) staining indicated the nuclei.
Expressional Analysis of GFP Tagged GCT Stromal Cells In Vivo
Balb/c nu/nu mice 6-7 weeks old female were used for this study and 100µl of injectate corresponding to 0.5x106-1.5x106 GFP-transfected GCT cells was injected into the sub-periosteum of the right tibia. The left tibia was injected with PBS to act as an internal control. Mice were monitored weekly for 5 weeks. The mice were sacrificed at day 8 or day 35 post-injection and stored in formalin. Both lower limbs were then removed for further analysis. We observed GFP expression in the injected limbs on days 8 and 35 (Fig. 3).
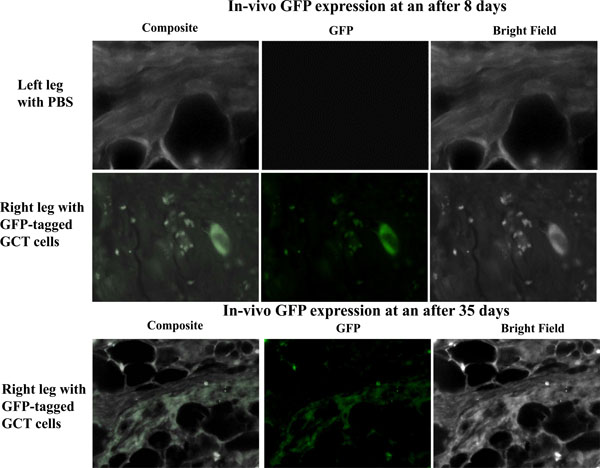
In vivo observation of GFP signals. We observed in vivo GFP expression on days 8 and 35 post injection in the subperiosteal region of the tibia of immunocompromised mice. No GFP signals were detected in tissue from the control contralateral leg.
DISCUSSION
Tumor progression and invasion is a complex biological process that involves remodeling of stromal tissue by invading cells. Giant cell tumors are normally benign with unpredictable behavior [16,17]. Although classified as a benign tumor of bone, GCT has been reported to metastasize to the lungs up to 5% of cases and in rare instances (1-3%) can be transformed to the malignant sarcoma phenotype with equal disease outcome [17-19]. The pathobiology of GCT is poorly elucidated due to lack of a GCT stable cell line and animal model to study tumor growth and physiology. We describe the generation and the utilization of a GCT cell line that stably expresses GFP both in vitro and in vivo.
GCT mesenchymal stromal cells became the homogeneous cell type whereas the multinucleated giant cells were eliminated from culture and maintained the phenotype up to the tenth passage were used for experiments [19, 20]. Here, we are describing gene transfer and stable cells generation in patient-derived primary GCT stromal cells. We were able to generate a stable GFP-tagged GCT cell line by an electroporation method. We detected GFP signals using fluorescence microscopy and western blot analysis at 52 weeks post transfection. Furthermore, we used this stable cell line in an in vivo mouse model and observed GFP signals by immunofluorescence microscopy after 8 and 35 days post-injection.
This model is the first described for GCT in which in vivo tracking of tumor viability can be successfully accomplished. Recently, a short-term in vivo model of GCT was reported using chick chorioallantoic membrane (CAM). This model was used to observe the dissemination of tumor cells along vessels leading away from the tumor [21]. GFP tagged GCT cells in an in vivo murine model would be a further step towards generation of a more physiologic in vivo model that could identify targets for systemic therapy in GCT.
In conclusion, we report stable gene transfection in GCT stromal cells that can survive and be visualized in vivo. The stable GCT stromal cell line described herein can serve as an excellent system for in vitro and in vivo therapeutic and mechanistic evaluation of molecular approaches to the clinical management of GCT.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
ACKNOWLEDGEMENTS
We would like to thank these following funding sources: Canadian Institutes of Health Research (CIHR) Grant, Hamilton Health Science New Investigator Fund, Hamilton Health Science Early Career Award, Juravinski Cancer Centre Foundation Grant, and McMaster University Surgical Associates Grant. The funders had no role in study design, data collect ion and analysis, decision to publish, or preparation of the manuscript.


