All published articles of this journal are available on ScienceDirect.
Safety of Intra-Articular Use of Atelocollagen for Enhanced Tissue Repair
Abstract
Collagen is an important biomaterial in intra-articular tissue engineering, but there are unanswered questions about its safety. We hypothesize that the addition of type-I-collagen for primary repair of the Anterior Cruciate Ligament (ACL) might result in a local and systemic reaction in a porcine model after 15 weeks as demonstrated by joint effusion, synovial thickening, elevated intraarticular and systemic leukocyte counts. Further, this reaction might be aggravated by the addition of a platelet concentrate. Eighteen porcine ACLs were transected and repaired with either sutures (n=6), a collagen sponge (n=6), or a collagen-platelet-composite (CPC; n=6). Twelve intact contralateral knees served as controls (n=12). No significant synovial thickening or joint effusion was seen in the collagen-treated knees. Synovial fluid leukocyte counts showed no significant differences between surgically treated and intact knees, and no differences were seen in leukocyte counts of the peripheral blood. The addition of a platelet concentrate to the knee joint resulted in lower serum levels of IL-1β, but serum levels of TNF-α were not significantly different between groups. In conclusion, the presence of collagen, with or without added platelets, did not increase the local or systemic inflammatory reactions following surgery, suggesting that Type I collagen is safe to use in the knee joint.
1. INTRODUCTION
There has been a recent upsurge in interest in using extracellular matrix-based materials to enhance repair of tissues within the knee joint. Specifically for the Anterior Cruciate Ligament (ACL), these materials have been used as platelet stabilization scaffolds in a central defect model [1, 2], as well as in a complete ACL transection model [3, 4] and also to enhance healing of an ACL reconstruction [5]. In those studies, these materials appear promising with regards to improving the biomechanical strength of the repairs. However, in vivo application of xenogenic biomaterials has consistently been accompanied by questions concerning the safety of such biomaterials [6].
There is a surprisingly high commonality in amino acid sequence and epitope structure for collagen across species, and, historically, the safety of the use of bovine and porcine collagen products, including Occlusin, TissueMend, Restore, and SurgiMend, has been well documented [7, 8]. The immuno-privileged nature of the avascular and alymphatic interior of the joint adds to the meaning of this beneficial safety record. Implantation of bovine collagen materials has been performed previously, particularly for indications such as hernia repair, wound healing, tendon reinforcement, plastic and reconstructive surgery, and staple line reinforcement [7]. These products have some similarities to our extracellular matrix-based scaffold, which has type I collagen as a major component, and some differences (Table 1). When used in clinical trials, previous products have been reported to be effective in supplementing soft tissue repair. Some of these materials are quickly resorbed by the host and replaced with scar tissue, while others are more permanent and may become encapsulated with fibrous material. Regardless of the permanence of the implant, all have been reported to play a significant role in enhancing the healing of the tissues in which they are implanted [7]. Despite widespread use of these materials, much of which has been outside the joint space, there have been no real reports of immune reactions. However, intra-articular application of collagen or another extracellular matrix-based scaffold might provoke reactions ranging from simple immunologic rejection to severe reactive arthritis. Furthermore, it is prudent to consider that the addition of a platelet concentrate could introduce a number of cytokines and consequently trigger, facilitate, or exacerbate an immunogenic response. In this study we seek to determine if there was an inflammatory response to a bovine-derived extracellular matrix-based scaffold within the privileged environment of the knee joint.
Summary of Currently Available Collagen Biomaterials Including their Application, Animal Source, Tissue Source, Method of Terminal Sterilization, Method of Cross-Linking, Material Name, and Manufacturer
| Application | Animal Source | Tissue Source | Terminal Sterilization | Cross-linking | Material | Manufacturer |
|---|---|---|---|---|---|---|
| Tendon Repair Reinforcement | Bovine | Dermis | Low temperature Ethylene Oxide | None | TissueMend | TEI Biosciences |
| Porcine | Dermis | E-Beam | None | Conexa | Lifecell | |
| Porcine | Small Intestine Submucosa | E-Beam | None | Restore | DePuy | |
| Porcine | Small Intestine Submucosa | Gamma Irradiation | Carbodiimide | CuffPatch | Organogenesis | |
| Rotator Cuff Repair | Human | Amniotic Membrane | Purion Process E-Beam | None | Rotator Cuff Patch | BioArthro |
| Porcine | Dermis | Gamma Irradiation | HMDI | Zimmer Collagen Repair Patch | Tissue Science Laboratories | |
| Skin and wound healing | Bovine | Dermis | Low temperature Ethylene Oxide | None | PriMatrix | TEI Biosciences |
| Porcine | Small Intestine Submucosa | Ethylene Oxide | None | Oasis | Cook Biotech | |
| Hernia Repair | Porcine | Dermis | Gamma Irradiation | HMDI | Permacol | Tissue Science Laboratories |
| Porcine | Dermis | Ethylene Oxide | EDC | CollaMend | Devol | |
| Bovine | Dermis | Low temperature Ethylene Oxide | None | SurgiMend | TEI Biosciences | |
| Staple-line reinforcement | Bovine | Pericardium | E-Beam | None | Peri-Strips | Synovis |
| Homologous Use | Human | Dermis | None | None | GraftJacket | Lifecell |
Abbreviations for Terminal Sterilization and Cross-linking Techniques: E-beam – Electron Beam; HMDI – Hexamethylene diisocyanate; EDC – 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide.
Any immunologic response might act on either or both of two levels. It might be local, confined within the barrier of the joint capsule, or systemic, affecting the whole body. The former would typically manifest itself with a combination of synovial hypertrophy, intra-articular effusion, and leukocytosis in the synovial fluid. The latter would present itself as leukocytosis and increase of inflammation markers in the peripheral blood.
The objective of this study was to assess the safety patterns of a bovine extracellular matrix-based biomaterial with and without addition of a platelet concentrate when used to augment primary repair of the ACL. We chose a 15 week time point to study indicators of both local and systemic reactions in a porcine model. To assess local reactions we defined three endpoints: joint effusion measured digitally on MRI and in physical examination, synovial hypertrophy assessed on histological specimens and on MRI, and leukocyte count in aspirates of the synovial fluid. We defined leukocyte counts in the peripheral blood as an endpoint representing systemic reactions. To assess the meaning of platelet-rich plasma (PRP) as a facilitator of immunological reaction we measured IL-1ß and TNF-∞ from the peripheral blood.
2. MATERIALS AND METHODS
2.1. Experimental Design
Approval from the Institutional Animal Care and Use Committee was obtained prior to the start of this study. Thirty-kilogram female skeletally immature 3 to 4-month-old Yorkshire pigs were used to minimize variability among animals. Prior studies have shown a stronger healing response in this population than seen in other age groups [9, 10]. 18 animals underwent ACL transection followed by one of three treatments: suture repair alone (SUTURE group; n=6), suture repair augmented with a collagen sponge (SPONGE group; n=6), and suture repair supplemented with a collagen-platelet composite (CPC) composed of a collagen sponge, collagen hydrogel and autologous platelet rich plasma combined before adding to the repair site (CPC group; n=6). Twelve contralateral knees which did not have surgery were analyzed as a control group (INTACT group; n=12). All endpoints were assessed at 15 weeks.
In our study we examined both systemic reactions (whole blood counts, cytokine release) and local reactions to the collagen implant. For local reactions, range of motion, synovial hypertrophy, synovial fluid leukocyte count, and joint effusion were considered. For these parameters, the contralateral intact knees served as the control for the surgical knee. For systemic parameters, we then compared groups of animals with the different surgical treatments – sutures alone, sponge, and collagen-platelet composite (CPC).
2.2. Collagen and Collagen-Platelet Composite (CPC) Preparation
The extracellular matrix-based scaffold (MIACH, Children’s Hospital Boston) was manufactured in our laboratory as previously described [11]). In brief, bovine connective tissue was solubilized and digested with pepsin. The resulting slurry was neutralized by mixing with HEPES Buffer (Cellgro, Mediatech, Inc., Herndon, VA), 5x PBS (Hyclone Laboratories, Logan, UT), and 7.5% sodium bicarbonate (Cambrex BioScience Walkersville, Inc., Walkersville, MD). To prepare the collagen sponges, this mixture was lyophilized until dry in 15ml tubes. For the CPC group, autologous platelets were prepared by centrifugation of autologous anticoagulated blood at 100 g for 15 min. This resulted in an approximate increase in platelet concentration from a range of 544,000 to 623,000 per mm3 (474,000±276,000 per mm3) to 1,630,000 to 3,638,000 per mm3 (2,097,000±1,120,000 per mm3), consistent with a 4±1-fold increase in platelet concentration. The platelet solution was added to the collagen slurry to keep the plasma: collagen ratio at 1: 1 for the collagen–platelet gels. Collagen pH was tested before addition of platelets to ensure a neutral pH. The mixture was kept on ice until use. At the time of surgery a collagen sponge or a collagen sponge soaked in the collagen-platelet gel was secured in the wound site of the ACL.
2.3. Surgical Procedures for Enhanced Primary Repair of the ACL
All procedures were performed under general anesthesia. Prior to surgery, blood was drawn for complete blood counts and PRP preparation, and range of motion, as well as a Lachman test, were documented. Both hind limbs were prepared with chlorhexidine and betadine, and sterilely draped. A median mini-arthrotomy was performed and the ACL was transected as previously described. Complete transection of the ACL was verified with a positive Lachman maneuver and visual assessment. Subsequently, knees were copiously irrigated with sterile saline, and surgical repair of the ACL was performed as follows. An absorbable suture anchor (TwinFix AB 5.0 Suture Anchor with DuraBraid Suture (USP#2); Smith and Nephew, Inc, Andover MA) was placed at the back of the femoral notch. Two #1 Vicryl sutures were placed into the tibial stump in a variable depth fashion to provide four limbs of suture for the repair. For the SUTURE group, the four sutures from the anchor were tied to the four sutures in the tibial stump to make a four-stranded repair. For the SPONGE group, a collagen sponge was threaded onto the sutures and then the four sutures tied. For the CPC group, the sponge was threaded onto the sutures and soaked in a collagen-platelet hydrogel and the sutures tied. In all groups, the sutures were tied in approximately 70 degrees of flexion or 30 degrees short of full extension. The knee was then closed in layers. Animals were not restrained postoperatively and returned to their cages for normal activity and nutrition ad libitum. One postoperative dose of 0.01 mg/kg IM of Buprenex and a Fentanyl patch 1 to 4 mg/kg were provided for postoperative analgesia. All animals were shipped to a supervised off-site research farming location 4 weeks postoperatively where they stayed until euthanasia.
2.4. Outcome Measures of Joint Effusion
The joint effusions were measured primarily using MRI. Under general anesthesia, in vivo magnetic resonance imaging was performed at 1.5 Tesla (GE Medical Systems, Milwaukee, WI) with an eight-channel phased array coil at the specified time points. Scanning was performed with the knees placed in maximum extension. Conventional MRI included multiplane T1, FSE PD and T2 weighted images. Field of view (FOV): 16-18 cm, matrix: 256x256, (repetition time/ echo time) TR/TE: 400/16, 2500/32, 3000/66 msec, echo train length (ETL): 8, bandwidth (BW): 15 kHz, slice thickness: 3, interslice gap: 1mm). Perfusion was evaluated by using the spoiled gradient echo sequence (TR/TE=200/2ms, flip angle=60, 3mm slice thickness, and 0.625mm in plane resolution) with an intravenous contrast agent (Magnevist; Berlex, Wayne, NJ) 0.2 ml/kg injected 10 s after the start of scan. Five images were obtained per slice, 78 s apart. Post contrast T1-weighted images were obtained (FOV: 16cm, matrix: 256x256, TR/TE: 400/9msec, slice thickness: 3mm, interslice gap: 1mm) in the coronal and sagittal planes. All images used for analysis were taken in the plane. The sagittal FSE T2 image displaying the largest cut of the patella was chosen and assessed using the ruler provided in Synapse version 3.1.1 (Fujifilm Medical Systems USA, Inc., Stamford CT). The fluid space between the top of the patella to the femur, as well as the extension of the fluid above the patella were measured (Fig. 1). The secondary measures of joint effusion were changes range of motion in flexion and extension under anesthesia from preoperative to 15 weeks. All measurements were taken by the same investigator who was blinded as to the treatment group. Maximum flexion was defined as the greatest angle obtained between femur and tibia with the hip maximally flexed to relax the quadriceps, and maximum extension was defined as the smallest angle obtained between femur and tibia with the hip maximally extended to minimize the contribution of the hamstrings.
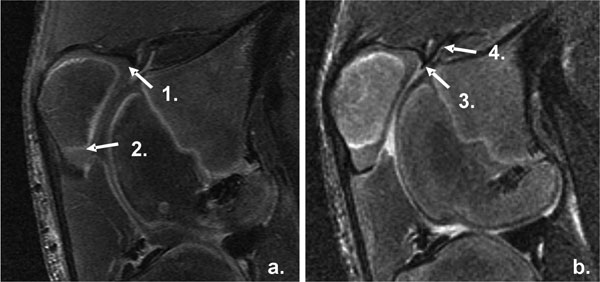
MRI Measurement of synovial thickness and effusion. Image a: SAG PG T1 Sequence 1. Suprapatellar Synovium. 2. Infrapatellar Synovium. Image b: FSE T2 Sequence 3. Suprapatellar Effusion Width. 4. Suprapatellar Effusion Length.
2.5. Outcome Measures of Synovial Hypertrophy
The primary measure of synovial hypertrophy was pathologic evaluation of the synovium at 15 weeks. The knees were fixed in neutral buffered formalin for 1 week, decalcified carefully over 24 to 72 h (DELTA-Cal, Delta Products Group, Aurora, IL) and the entire knee cut longitudinally at 7µm in a sagittal plane passing through the ACL, embedded in paraffin, and stained with hematoxylin and eosin. Sections were evaluated independently and in duplicate by two blinded observers. The synovial tissue covering the healing ACL (ACL-Synovium) and lining the joint capsule (CAPS-Synovium) at a location remote from the prior incisions, were fixed in formalin and sectioned. Three areas of each tissue were analyzed. Within each area, the number of cell layers comprising the synovium was recorded, and vascularity, lymphocytes, and villi were rated on a zero-to-three scale, with zero being none present, one being below normal, two being normal, and three being above normal. Secondary measures of synovial hypertrophy were the thickness of the synovium and capsule together on MRI in the suprapatellar and infrapatellar regions. Synovial thickness was measured above and below the patella on the SAG PG T1 sequence.
2.6. Outcome Measures of Synovial Fluid Leukocyte Count
At the 15 week time point, synovial fluid was aspirated from the knees using a 20G needle. The white blood cell count of the undiluted synovial fluid was determined using a VetScan HM2 (Abaxis, Union City, CA).
2.7. Outcome Measures of Systemic Inflammatory Markers
Pre-operative systemic leukocyte counts were compared to corresponding values at 15 weeks after atelocollagen implantation. Blood was drawn through a large bore needle (18g or larger) into tubes containing Anticoagulant Citrate Dextrose Solution (Cytosol Laboratories, Inc., Braintree, MA) and the systemic white cell count determined using a VetScan HM2 (Abaxis, Union City, CA). Additional blood was drawn into serum separator tubes and allowed to clot overnight. Aliquots of porcine serum were collected and stored at -80°C until further processing. Serum levels of IL-1β and TNF-α were measured using commercially available ELISA kits (Human IL-1 beta/IL-1F2 Quantikine ELISA Kit, Porcine TNF-alpha/TNFSF1A Quantikine ELISA Kit, R&D Systems, Minneapolis, MN, USA).
2.8. Statistical Analyses
Statistical comparisons for both the local and systemic reaction parameters were made between all treatment groups. Differences between groups were compared using ANOVA with Bonferroni adjustment for subgroup analyses for continuous outcomes. χ2 tests were used for categorical outcomes. Statistical analyses were performed using StatView software (version 5.0.1, SAS Institute Inc., Cary, NC) or intercooled Stata 10 (StataCorp LP, College Station, TX). A p-value of p < 0.05 was used as criterion for statistical significance.
3. RESULTS
All animals tolerated the procedures well and were full-weight bearing within one week. One animal in the SPONGE group was euthanized shortly after surgery due to complications unrelated to the surgery, leaving a group size of 5 animals for that group and a group size of 11 in the INTACT group. All other animals had an uneventful postoperative recovery period and no complications during the 15 weeks of follow-up.
The addition of atelocollagen in either SPONGE or CPC-GEL form had no effect on the amount of suprapatellar joint effusion width as measured on the sagittal sections of MRI compared to INTACT knees (p = 0.201, Fig. 2). Knees treated with suture repair alone had a significantly lower suprapatellar effusion length than either the INTACT or CPC knees (p < 0.001 for both comparisons; Fig. 2), though there was no significant difference between the INTACT and CPC-treated knees. There was also no difference in extension of knees exposed to atelocollagen when compared to those not exposed to atelocollagen at fifteen weeks post-operatively (Fig. 3, p > 0.4762 for all comparisons). Interestingly, there was a greater loss of flexion in the SUTURE group when compared with the INTACT (p = 0.0034), SPONGE (p = 0.0053), and CPC (p = 0.0077) groups (Fig. 3).
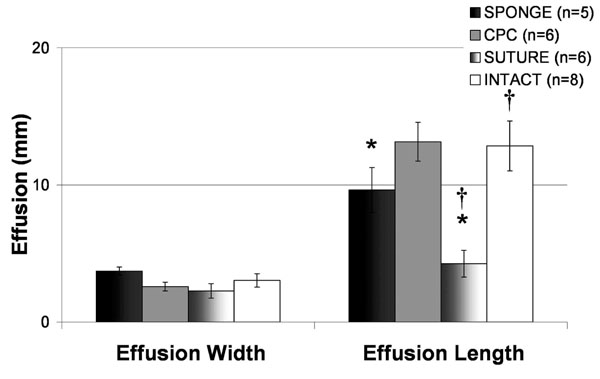
Comparison of Suprapatellar Effusion Dimensions across Treatment Groups. The addition of atelocollagen in either SPONGE or CPC-GEL form had no effect on the amount of suprapatellar joint effusion width as measured on the sagittal sections of MRI compared to INTACT knees (p = 0.201). Knees treated with suture repair alone had a significantly lower suprapatellar effusion length than either the INTACT or CPC knees (p<0.001). All values represent mean effusion (mm) +/- standard error.
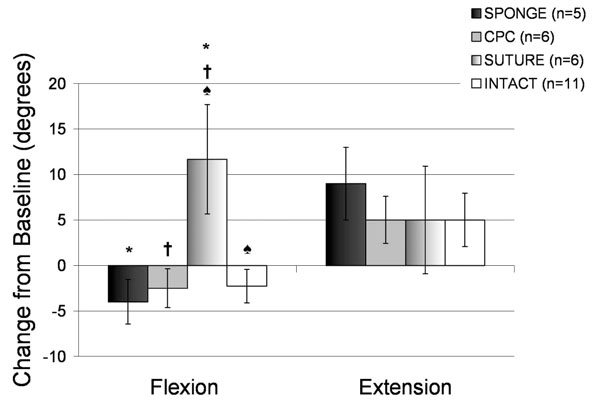
Physical Examination at 15 weeks post-operatively for all treatment groups. All values represent changes in these parameters from the baseline measurements at the time of surgery. Positive values indicate an increase in flexion or extension from baseline. Negative values indicate a loss of flexion or extension from baseline. Error bars represent standard error. * indicates a significant difference between the SPONGE and SUTURE groups (p < 0.01); † indicates a significant difference between the CPC and SUTURE groups (p < 0.01); and ♠ indicates a significant difference between the INTACT and SUTURE groups (p < 0.01).
Infrapatellar synovial thickness was not significantly different when comparing suture and intact groups (p=0.9063). The difference between the CPC and Sponge groups approached significance (p=0.0665), and all groups showed no significant differences when compared to intact knees (p>0.1, Fig. 4). The histological assessment showed no difference in the numbers of cell layers at the site of the ACL scar (p=0.255) and the synovium at a site that was unaffected by the procedure (p=0.231). (Table 2) Also, there were no synovial villi in either group (p=0.347). Finally, there were no significant differences in the vascularity between groups (p=0.862) and no lymphocytic infiltrates based on treatment with collagen (p=0.441).
Qualitative Histological Evaluation of CPC and Suture Groups
| Cell Layers | Lymphocytes | Vascularity | Villi | |
|---|---|---|---|---|
| CPC: ACL-Synovium (n=7) | 2.88 ± 0.89 | 0.05 ± 0.08 | 1.33 ± 0.71 | 0.02 ± 0.02 |
| CPC: CAPS-Synovium (n=7) | 3.48 ± 0.96 | 0.00 ± 0.00 | 1.24 ± 0.64 | 0.07 ± 0.07 |
| SUTURE: ACL-Synovium (n=6) | 3.33 ± 1.054 | 0.06 ± 0.14 | 1.42 ± 0.74 | 0.06 ± 0.09 |
| SUTURE: CAPS-Synovium (n=6) | 2.94 ± 1.58 | 0.08 ± 0.14 | 1.22 ± 0.72 | 0.11 ± 0.20 |
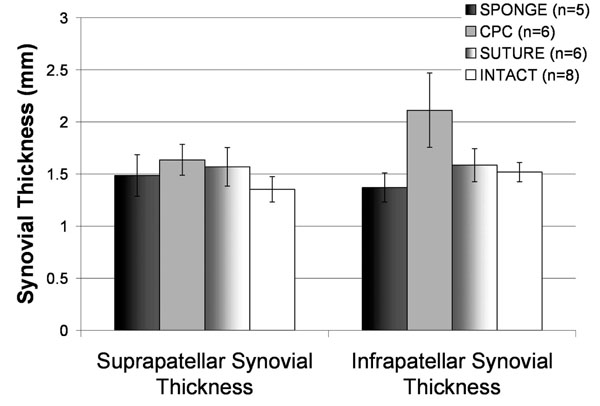
Synovial thickness as measured by MRI at 15 weeks post-operatively for all treatment groups. Infrapatellar synovial thickness was not significantly different when comparing suture and intact groups (p=0.9063). The CPC and Sponge groups approached significance (p=0.0665), and all groups showed no significant differences when compared to intact knees (p>0.1). All values represent changes in these parameters from the baseline measurements at the time of surgery. Error bars represent standard error.
The synovial tissue covering the healing ACL (ACL-Synovium) and lining the joint capsule (CAPS-Synovium) at a location remote from the prior incisions, were fixed in formalin and sectioned. Three areas of each tissue were analyzed. Within each area, the number of cell layers comprising the synovium was recorded, and vascularity, lymphocytes, and villi were rated on a zero-to-three scale, with zero being none present, one being below normal, two being normal, and three being above normal. All values represent mean +/- standard deviation. There were no observed significant differences between the groups treated with suture alone and those treated with the collagen-platelet composite for any of the measures.
The assessment of the leukocyte count in the synovial fluid showed no significant difference in any of the treatment groups or the INTACT knees at 15 weeks (p=0.326). In all treated knees, the WBC count remained below 2.4x10^3/ml (2.4x10^3/ml, INTACT; 1.5x10^3/ml, SUTURE; 0.8x10^3/ml, SPONGE; 0x10^3/ml, CPC). There was also no significant elevation of systemic leukocyte counts in the peripheral blood over the course of the experiment (p = 0.154). All values remained within normal limits throughout the experiment.
Serum levels of IL-1β were lower in the group exposed to PRP than in the unexposed group with values of 3.7 ± 0.3 pg/ml and 8.0 ± 1.1 pg/ml, respectively. This difference was statistically significant (p = 0.002). Levels of TNF-α were 165.6 ± 20.9 pg/ml in the group exposed to PRP and 151 ± 10.4 pg/ml in the unexposed group, and were not significantly different (p = 0.836).
4. DISCUSSION
The use of collagen, mostly of bovine and porcine origin, as a biomaterial for intra-articular tissue engineering applications in orthopedic surgery has shown much promise in vitro and in vivo [12-14]. However, there still are concerns being uttered regarding the safety of such applications, and, to date, there is a lack of evidence to counter such claims. Collagen is highly similar in the amino acid sequence and epitope structure across species and generally considered safe [15]. The incidence of immunologic reactions to xenogenic collagenous implants ranges between 2-4% in clinical observation of extra-articular application of bovine and porcine collagen and these reactions mostly are trivial and inconsequential [16, 17]. It is to be expected that these observations overstate the incidence of intra-articular reactions, which should be lower due to the immuno-privileged nature of the intra-articular environment. It was the objective of this study to test for clinically relevant, adverse reactions to the intra-articular use of a collagenous biomaterial, more specifically atelocollagen, in an in vivo model. Additionally, we included a platelet concentrate as a cofactor into our model, since most intra-articular current tissue engineering applications strive to harness the regenerative powers of such agents.
Atelocollagen is considered an even safer alternative to generic collagen, because the telopeptides of the collagen molecule, which are thought to be the major agent of immunogenicity, are removed [6, 18]. However, it has been shown that, in addition to the telopeptides, central and helical domains of collagen play an important role in the immunologic interaction between bovine and porcine collagen and human host tissue as well [6, 19]. It should be considered that any such interaction might be due to either antigenicity, which is the interaction between collagen and pre-existing antibodies, or immunogenicity, which involves the de-novo formation of antibodies against specific epitopes. The latter might also result in an auto-immune response if similar epitopes as presented by the implant are also presented in host tissues too. This process is the bedrock of collagen-induced arthritis (CIA), an animal model of rheumatoid arthritis [20, 21]. In CIA, animals are injected with collagen emulsified in an immunological adjuvant – a reagent that boosts immune responses [22]. Fears of initiating CIA by using collagen implants in patients, however, are ungrounded because CIA requires at the very least an adjuvant, and – despite investigators’ best efforts - has thus far only been accomplished for types II and XI collagen in a limited number of species [6, 20, 22]. There remains, however, the risk of an immune reaction of unspecified severity towards a collagenous implant itself. Thus it was the objective of this study to explicitly assess such potential reactions in a relevant animal model. Any such reaction might be facilitated or exacerbated by cytokines secreted after the addition of PRP, and this factor was included as covariate in our study. Finally, we chose a time point at 15 weeks to make sure that none of any observed effects are caused by perioperative events.
Our study has some potential limitations. First of all, our definition of an adverse immunological reaction is rather non-specific, but since type and extent of a possible host reaction to a collagen implant cannot be reliably predicted we deliberately chose these more general endpoints. Of note, the objective of this study was to search for adverse effects that might affect clinical outcome due to rejection, inflammation, adhesions, et cetera, allowing for more in depth research into any found reaction later on. By no means did we intend to give a complete, comprehensive, and chronological description of all immunological processes involved in the intra-articular use of collagen. External validity is a final concern. Although the pig is a validated model of human immunity [23, 24], there might be some relevant differences in the interaction of a bovine collagen with a porcine versus a human host [6]. Additional studies in humans will be necessary to definitively confirm our conclusions.
The introduction of atelocollagen into the knee joint as either a sponge or platelet gel to stimulate healing of the ACL did not appear cause a significant joint effusion when compared to intact knees. Interestingly, there were some significant differences between those knees treated with sutures alone and intact knees, on outcomes scales, MRI, and range of motion. However, the rather small absolute values of these differences minimize their clinical importance. There was a significant difference in the effusion length of the suture group and intact group, with the suture group having a smaller effusion length than the intact group (Fig. 2). One possible explanation for this could be the efflux of synovial fluid through the femoral or tibial tunnels into the extraarticular space, therefore decreasing the effusion length of the suture group. The femoral tunnel of those in suture group is perhaps less obstructed than those of other groups (intact animals are without a femoral tunnel; collagen may block the femoral tunnel of other groups) which may allow for increased fluid passage in this particular group. It is also possible that the failure of healing of the ACL in the suture group led to increased stress on the secondary capsular structures, resulting in hypertrophy and contracture of the capsule and elimination of some of the normal joint space. Future studies on the mechanism behind this observed effect are planned.
Synovial reaction and inflammatory changes in the synovium may be more sensitive markers of the immunogenic process than joint effusion. We found no evidence that the addition of a collagen-platelet composite affected the thickness of the synovium or was associated with the development of synovial villi. Nor did we observe increased vascularity or lymphocytic infiltrates when comparing ACL treated with a collagen-platelet composite with those treated with suture repair alone. Other materials that caused immunologic rejection when previously used for ACL replacement, such as Carbon Fiber Ligament [25-27], Goretex [28-30], or Kennedy LAD [31] showed considerable to massive monocytic infiltrates.
No differences were seen in synovial fluid between knees or in systemic leukocyte counts over time to suggest significant humoral effects occurring as a result of the implantation of atelocollagen. Contrary to effusion and synovial thickening, leukocyte counts are able to reflect even minor immunological reactions. Although such low-grade reactions might not cause further immune responses, they might very well affect the outcome of the procedure and thus might be a more important endpoint than joint effusion since they are independent from biomechanics. Quite the contrary, any synovial leukocytosis is a highly specific and sensitive sign for intra-articular immunological processes.
We wanted to address the question of whether addition of a platelet concentrate would affect potential immune responses. Platelets, oftentimes in the form of platelet rich plasma, have shown much promise as stimulators of regenerative processes. However, the amply released cytokines that cause the beneficial effects of platelet concentrates might also provoke or amplify immune responses. Despite previous findings of an association between increased platelet concentration and cytokine release [32], we did not observe an association between any of the endpoints and the presence or absence of PRP. TNF-α levels were similar in both the PRP-exposed and non-exposed groups. However, TNF-α has been reported to be released from platelet-rich fibrin matrices [33]. Therefore, in the knees where PRP was added, an intrinsic decrease in TNF-α expression by the synoviocytes may have been masked by the TNF-α release from the PRP. Further experiments to determine the geography of TNF-α release from an injured joint are planned.
While we did not observe systemic differences in TNF-α between PRP and non-PRP groups, animals treated with PRP were found to have significantly lower levels of IL-1β than those not treated with PRP. These findings are consistent with prior reports that have shown that PRP does not have a significant effect on TNF-a release [34], but that PRP does play a role in the suppression of IL-1β release from chondrocytes [35]. In addition, IL-1β production is stimulated by TNF-α, it is possible that a later increase in IL-1β may follow an earlier increase in TNF-α at time points not studied here. Additional time points and further research are planned to investigate this hypothesis.
In addition, a limitation of this study was that we did not look at the cytokine levels of the synovial fluid inside the joint. Future studies are planned to evaluate this in more depth given the interesting findings here, including the lower synovial fluid WBC in the CPC group. While it can not be stated for certain why the WBC count in the joint was lower for the CPC group than the INTACT group, prior reports state that PRP suppresses the inflammatory cascade [36] and therefore might have reduced WBC extravasation, a process we are interested in exploring further.
In conclusion, the use of either a collagen sponge or gel made little difference in the resulting changes induced in the joint or surrounding tissues. The reduction of IL1-β with the use of PRP suggests the possibility of a decrease in the occurrence of inflammation with enhanced repair, a preliminary finding that deserves additional study to determine whether suture repair enhanced with a collagen-platelet gel may lead to decreased inflammation in the knee when compared with suture repair alone. These findings suggest that the use of Type I soluble collagen is relatively safe, although additional safety studies in humans will be required if these techniques prove efficacious in animal models.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Arthur Nedder, Mark Kelly, Maria Valenza, Matthew Palmer, and Eduardo Abreu for their assistance with this project. In addition, funding was received from NIH grants AR054099 (MMM).
CONFLICT OF INTEREST
Declared none.


