All published articles of this journal are available on ScienceDirect.
Early Changes in Bone Specific Turnover Markers During the Healing Process After Vertebral Fracture
Abstract
Background:
The present study measured longitudinal changes in bone turnover markers in elderly patients with vertebral fracture and investigated the relationship among bone turnover markers, duration of bed rest and bone mineral density (BMD).
Methods:
Criteria for patient selection were 50 years in age and older, and presence of VF. Serum bone-specific alkaline phosphatase (BAP) was measured as a marker of bone formation. Urinary crosslinked N-terminal telopeptides of type I collagen (NTX) was measured as a marker of bone resorption. In principle, samples were collected just after injury, within 24 h, and 1, 2, 3, 5 and 8 weeks after. We also measured duration of bed rest and BMD.
Results:
The study population consisted of 42 cases. The average BMD of the lumbar vertebrae was 0.670 ± 0.174 g/cm2. Bed rest period was 17.9 ± 8.8 days. BAP showed significantly higher values at 2 and 3 weeks compared with the baseline value. Thereafter, BAP progressively decreased until 8 weeks. Urinary NTX was increased soon after the onset of pain with the same patterns in BAP. Urinary NTX values reached a peak at 3 weeks, and then they kept significantly higher values until 8 weeks. The peak value of serum BAP was affected by the duration of bed rest, although that of the urinary NTX was not. The peak values of serum BAP and urinary NTX showed negative correlations with the initial BMD values.
Conclusions:
Bone turnover markers remained higher at 8 weeks, even patients symptom was healed after VF. Bone turnover markers were affected on physical activity and BMD.
Osteoporosis is a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture [1]. Vertebral fractures (VF) are the classic hallmark of osteoporosis [2].
Generally, it is thought that bone loss accelerates with high turnover of the bone after menopause. This accelerated turnover declines over time until a steady state of low turnover is eventually reached. Further, some studies have indicated that there is high turnover in the early postmenopausal period that decreases through the seventh and eighth decade, over 15 years after menopause [3, 4]. Recently, we reported that bone turnover markers associated with back pain (BP) are significantly increased in elderly women, and speculated that these bone turnover markers are increased by unrecognized VF in patients with BP [5].
Several clinical studies have pointed out that bone turnover markers after fractures increase with fracture healing [6-15]. Usually, VF requires prolonged bed rest for pain relief. Bone resorption markers are strongly related to physical activity [16-20]. Therefore, VF or low physical activity such as bed rest might differently influence bone turnover markers. However, there have been no reports regarding the relationship between bed rest after VF and bone turnover markers. Longitudinal and prospective studies are desirable to elucidate the time course of changes in bone turnover markers in elderly women and the precise relationships between bone turnover markers, BP, and VF.
In our institute, elderly patients with severe BP were evaluated using radiograph (X-P), magnetic resonance imaging (MRI) and bone scintigram (BS). Many of these patients were diagnosed as having VF and were treated with bed rest [21]. The present study monitored longitudinal changes in bone turnover markers in elderly patients with VF and investigated the relationship among bone turnover markers, duration of bed rest, and bone mineral density (BMD).
MATERIALS AND METHODOLOGY
Patients
Criteria for patient selection were 50 years of age and older and the presence of osteoporotic VF with or without a history of minor trauma. Patients with high-energy injuries were excluded from this study. Forty-two consecutive patients with VF combined with osteoporosis were admitted to our institution because of severe pain when changing positions and inability to sit up. All the patients gave informed consent for examination and medical treatment. This study was carried in accordance with the Declaration of Helsinki. The study was approved by the Ethics Committee of Suwa Red Cross Hospital. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Radiologic Evaluation
Patients underwent a series of imaging studies including anterior-posterior (AP) and lateral X-P, MRI of the spine, and BS within one week after admission.
Diagnosis of VF on MRI was made when T1-weighted images showed low intensity images in the involved vertebra on the sagittal plane and intense radiotracer uptake on BS. In addition, BMD of the AP lumbar spine was measured using dual X-ray absorptiometry (DXA) (QDR2000 Hologic, Waltham, MA, USA) for unaffected vertebra.
The final diagnosis of VF was based on radiographic findings on MRI and BS, and the clinical course of the patient.
Treatment of Osteoporotic VF
Patients were treated conservatively with bed rest until severe BP had diminished and patients could sit up with mild, tolerable BP. The duration of bed rest was defined as the period from the onset of VF to the time patients could ambulate with external fixation by orthosis or plaster cast. During this period, the patients were allowed to raise in bed if doing so without pain. Patients who could sit up with no pain were allowed to walk with external fixation by orthosis or plaster cast.
Biochemical Markers
Serum bone-specific alkaline phosphatase (BAP) was measured as a marker of bone formation. Urinary level of cross-linked N-terminal telopeptides of type I collagen (NTX) was measured as a marker of bone resorption by enzyme-linked immunosorbent assay (ELISA). In principle, samples of venous blood and spot urine were collected six times as closely as possible in accordance with the following schedule: just after, within 24 h, and 1, 2, 3, 5, and 8 weeks after injury. Patients were encouraged to undergo measurements more than four times. Second spot urine samples were collected in the morning, to avoid first morning urine. The samples were stored at -20°C until analysis. The immunoassay was performed by SRL Inc. (Tokyo, Japan).
Statistical Analysis
The results are expressed as the means and standard deviation (SD). Differences in the levels of bone turnover markers between at the onset of BP and at each examination point were assessed using Student’s unpaired t-test. Significance was accepted at a p-value of less than 0.05. The correlations between bone turnover markers and period of bed rest or BMD were assessed using Pearson’s correlation coefficient.
RESULTS
The study population consisted of 42 cases, 28 women and 14 men, ranging in age from 57 to 95 years (mean, 77.7 years). The average BMD of the lumbar vertebrae was 0.670 ± 0.174 g/cm2 (Table 1). Bed rest period was 17.9 ± 8.8 days for patients with VF. All patients showed a low T1-weighted signal of the affected vertebra on MRI and intense radiotracer uptake on BS. All patients demonstrated a decrease in BP with conservative treatment.
Characteristics of patients
| Characteristics | Number or Mean ± SD (Range) |
|---|---|
| Male sex (Female) | 14 (28) |
| Age (years) | 77.7 ± 8.2 (57-95) |
| Weight (kg) | 51.0 ± 9.7 (35-75) |
| Height (cm) | 154.7 ± 7.3 (140-173) |
| Lumbar spine BMD | 0.670 ± 0.174 (1.073-0.409) |
The Time Course of Serum BAP Value (Fig. 1)
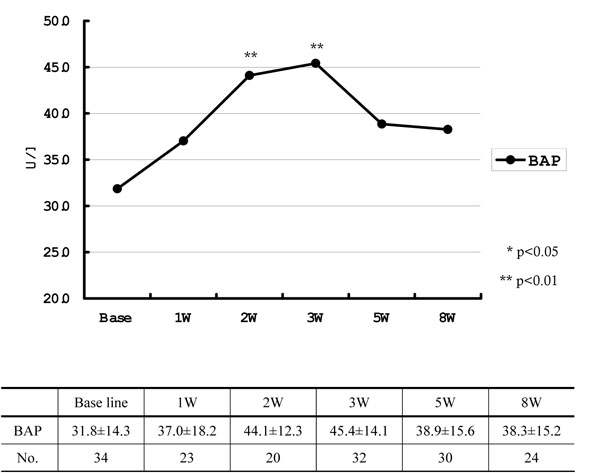
Baseline values and longitudinal absolute mean value (±SD) of serum BAP.
BAP was 31.8 ± 14.3 U/l at the baseline, which quickly increased to reach 45.4 ± 14.1 U/l at 3 weeks after the onset of BP, showing significantly higher values at 2 and 3 weeks compared with the baseline value. Thereafter, BAP decreased to 38.3 ± 15.2 U/l at 8 weeks. Although the difference was not significant compared to the baseline value, BAP continued to show slightly higher values until the 8th week.
The Time Course of Urinary NTX Value (Fig. 2)

Baseline values and longitudinal absolute mean value (±SD) of urinary NTX.
Urinary NTX was increased soon after the onset of BP, with the same patterns as for BAP. At 2 weeks after the onset of BP, urinary NTX rose from 73.2 ± 52.4 to 131.3 ± 77.9 nmol BCE / mmol Cr. Urinary NTX reached a peak at 3 weeks, and then maintained significantly higher values compared with the baseline until the 8th week. Both serum BAP and urinary NTX reached peak values 3 weeks after trauma, suggesting bone formation and resorption increase after fracture.
Effects of BMD and Bed Rest Period on Biochemical Markers
In these patients, the relation between the peak values of bone turnover markers, the duration of bed rest, and BMD were examined. There was no correlation between BMD and bed rest period. The peak value of BAP, however, had weak correlation with the duration of bed rest (Fig. 3): as the rest period became longer, the BAP value became significantly lower. In contrast, the peak value of urinary NTX did not correlate with the duration of bed rest (Fig. 4).
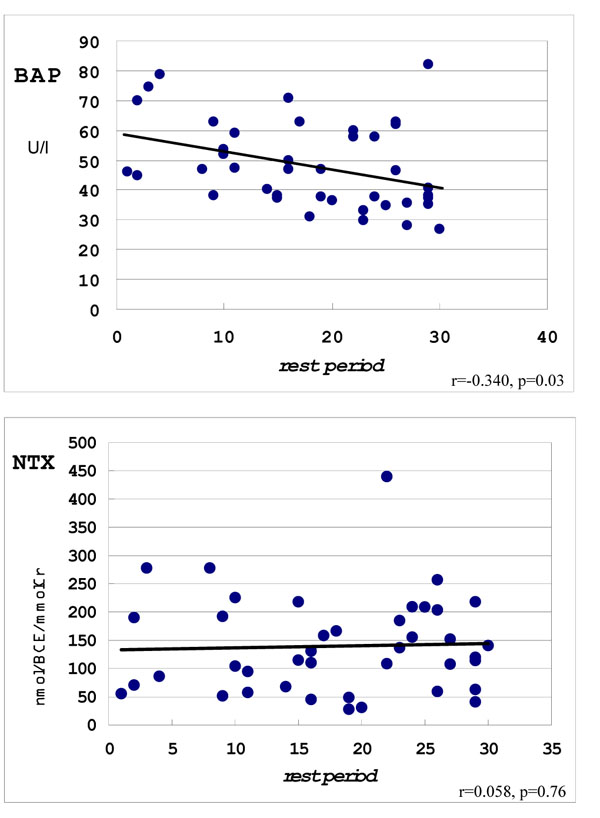
It is not correlation between NTX, but correlation between NTX and duration of bed rest.
The peak values of both serum BAP and urinary NTX showed negative correlation with the BMD value at the onset of BP (Figs. 5, 6).
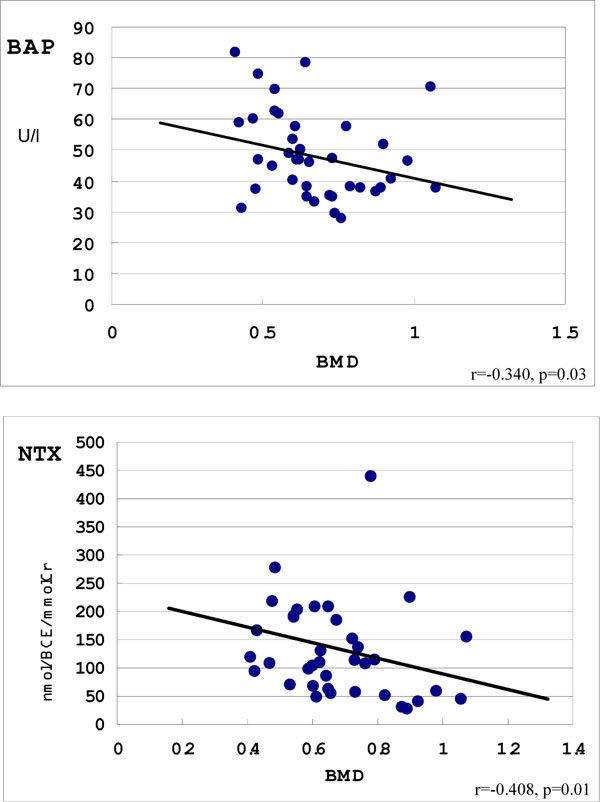
There was a correlation between BAP and BMD, as well as a correlation between NTX and BMD.
DISCUSSION
VF is a common osteoporotic fracture in elderly patients, and the incidence of VF has increased in recent years. In patients with osteoporosis, VF frequently causes BP [21-23]. As BP usually ceases within a few weeks, these fractures are not considered to induce severe clinical problems in most cases [24]. Furthermore, Vogt et al. reported that the fractures are recognized and diagnosed in only one third of patients with VF [25]. However, regardless of the presence of trauma, severe BP in patients with osteoporosis tended to indicate the presence of VF [21]. In patients with osteoporosis, VF causing severe BP requires a prolonged bed rest period for pain relief regardless of traumatic episode and/or vertebral deformity. Osteoporotic VF demonstrates various interesting clinical features, and such fractures may variously influence bone turnover markers.
Various biochemical markers reflect bone metabolism. When bone resorption occurs, breakdown products of type I collagen are released into the circulatory system. NTX is one of these breakdown products and the urinary NTX level has been reported to be a specific and sensitive marker of bone resorption [26]. BAP is a specific product of osteoblasts and is used as a typical bone formation marker to evaluate bone turnover. In the present study it was shown that both bone formation and resorption markers increased over 8 weeks after fracture.
Many authors have reported that bone turnover markers increased after fractures [6-15]. For example, hip fracture is one of the most common fractures in osteoporotic patients, comprising mainly femoral trochanter and neck fracture. Despite differences in post-operative therapy, the change of bone turnover markers during the healing of these fractures showed a similar pattern [13, 27]. However, the increase of bone turnover markers was significantly greater in trochanter fracture than in neck fracture, indicating that each type of fracture showed a different amount of influence on bone turnover markers.
In this study, patients with osteoporotic VF showed a tendency toward a negative correlation between bone turnover markers and BMD. It was suspected that mechanical force caused a larger area of vertebral body destruction for the patients with lower BMD, resulting in stronger effect on the bone turnover markers.
Generally, it is considered that the fracture may have a direct impact on bone formation and immobilization following fracture may induce an increase in bone resorption [7]. According to the previous studies, bone resorption markers are strongly related to physical activity [16-20]. In fact, some authors have found that bone resorption markers were increased [16-20] while bone formation markers decreased with bed rest [17]. Therefore, decreased physical activity such as bed rest usually causes an increase in bone resorption and an inhibition of bone formation. However, there are no reports describing changes in bone turnover marker due to osteoporotic VF with consideration for the duration of bed rest.
The present study demonstrated that the peak value of bone formation marker correlated with the duration of bed rest, while the peak value of bone resorption marker did not. It was considered that these correlations of bone formation marker were influenced by mechanical load to VF or physical activity. During fracture healing, acceleration of bone fusion might increase both bone formation and resorption markers. However, long-term bed rest accelerated bone resorption and decreased bone formation. For these reasons, it was speculated that early mobilization of patients increases mechanical load of VF before healing, accelerating the fracture healing process and increasing bone formation. Another possible reason is that long-term bed rest induces greater decline in bone formation; however it must be emphasized that the level is not significant, and further research is required.
This study has some limitations. First, the study was not population-based and the sample size was not large enough to draw definite conclusions. Second, not all data was collected in each period, and therefore time course of bone turnover markers might not be accurately reflected.
Recently, many studies have shown that an increase in bone turnover after menopause persists for longer than was previously believed. An increased incidence of fractures with aging has been demonstrated [28, 29]. Women aged over 80 years frequently have VF and the presence of such fractures is a strong risk factor for subsequent fractures [21, 25, 30, 31]. We previously reported that NTX and BAP levels were increased significantly in women over 80 years compared to those at age 60, and specifically in patients with BP associated with unrecognized fracture [5]. Considering these findings, elderly women with osteoporosis might show repeated VF regardless of symptoms such as BP, and VF might obviously affect the level of bone turnover markers. Therefore, in elderly patients with osteoporosis, bone turnover markers may be unstable.
CONCLUSION
Bone turnover markers remained higher at 8 weeks, even in patients for whom symptoms had ceased after VF. Bone turnover markers were likely to be affected by physical activity and BMD. An increase in bone turnover markers after vertebral fracture and the subsequent decrease may reflect bone formation and remodeling during the fracture healing process. High turnover may reflect not only bone fragility but also fracture healing.


