All published articles of this journal are available on ScienceDirect.
Adult Mesenchymal Stem Cells and Cell Surface Characterization - A Systematic Review of the Literature
Abstract
Human adult mesenchymal stem cells (MSCs) were first identified by Friedenstein et al. when observing a group of cells that developed into fibroblastic colony forming cells (CFU-F). Ever since, the therapeutic uses and clinical applications of these cells have increased research and interest in this field. MSCs have the potential to be used in tissue engineering, gene therapy, transplants and tissue injuries. However, identifying these cells can be a challenge. Moreover, there are no articles bringing together and summarizing the cell surface markers of MSCs in adults. The purpose of this study is to summarize all the available information about the cell surface characterization of adult human MSCs by identifying and evaluating all the published literature in this field. We have found that the most commonly reported positive markers are CD105, CD90, CD44, CD73, CD29, CD13, CD34, CD146, CD106, CD54 and CD166. The most frequently reported negative markers are CD34, CD14, CD45, CD11b, CD49d, CD106, CD10 and CD31. A number of other cell surface markers including STRO-1, SH2, SH3, SH4, HLA-A, HLA-B, HLA-C, HLA-DR, HLA-I, DP, EMA, DQ (MHC Class II), CDIO5, Oct 4, Oct 4A, Nanog, Sox-2, TERT, Stat-3, fibroblast surface antigen, smooth muscle alpha-actin, vimentin, integrin subunits alpha4, alpha5, beta1, integrins alphavbeta3 and alphavbeta5 and ICAM-1 have also been reported. Nevertheless, there is great discrepancy and inconsistency concerning the information available on the cell surface profile of adult MSCs and we suggest that further research is needed in this field to overcome the problem.
INTRODUCTION
About 130 years ago, the German pathologist Cohneim proposed the existence of non-hematopoietic stem cells in the bone marrow. He suggested that these cells could contribute to wound healing as they can be a source of fibroblasts [1]. Later, Friedenstein et al. identified human adult mesenchymal stem cells when observing a group of cells that developed into fibroblastic colony forming cells (CFU-F) [2]. Friedenstein provided strong evidence for the self-renewal potential of stem cells by demonstrating their ability to regenerate heterotropic bone tissue. These findings have been confirmed and expanded by many further laboratory studies which have shown that the cells isolated by Friedenstein can also be found in human bone marrow and could differentiate into a range of different mesenchymal lineage cells including chondrocytes, adipocytes, myoblasts and osteoblasts [1, 3-6].
Simmons et al. found that stromal cells supporting hematopoiesis were different to hematopoietic cells by showing that sexmismatched HLA-identical cells from patients solely expressed the host genotype [7]. This again supports Friendenstein’s findings showing hematopoieticstem cells were physically different from transplanted sexmismatched cells capable of heterotropic osseous tissue formation [8]. Now that these cells are found to be different, research is being done to identify whether there exist specific cell surface antigens to identify phenotypic differences between mesenchymal and hematopoietic stem cells. Even though many mesenchymal stem cell surface antigens have been cultured, there have been very few in vivo phenotypic characterization of MSCs [9]. Bianco et al. conducted one of the first studies with the intention of characterizing MSC-like cells both histologically and phenotypically. Interestingly, it has been shown that a wide range of non-hematopoietic stem cells exist in the bone marrow and that MSC are merely a subset of this population. These include “multipotent adult progenitor cells” (MAPCs), “endothelial progenitor cells” (EPCs), “marrow-isolated adult mutilinease inducible cells” (MIAMI), “ very small embryonic-like stem cells” (VSELs) [9-13].
Crisan et al. has found that multipotent mesenchymal stem cells exist in many different human organs [14]. To support this fact, it has been shown that MSCs are not confined to bone marrow and can also be found in placenta, dental pulp, tendons, skeletal muscle, fat, umbilical cord blood and amniotic fluid [14-22]. Despite the common belief that mesenchymal stem cells are derived from the embryonic mesoderm, a recent study by Takashima and colleagues showed that the earliest lineage of MSC-like cells are developed, at least in part, from Sox1(+) neuroepithelium through a neural crest intermediate stage rather than from mesoderm [23]. These cells have been shown to be replaced by MSCs in later development. Nagoshi and colleagues have recently demonstrated that neural crest-derived cells migrate through the bloodstream to the bone marrow [24]. These cells are present in the bone marrow and can develop into myofibroblasts, glial cells and neurons. Further research is needed to assess whether there is a link between cells indentified by Friedenstein and those identified by Takashima and colleagues [25].
Various studies have demonstrated the potential use if MSCs in gene therapy, transplants due to their special immunogenic properties and tissue injuries. Current research aims at characterizing, expanding and identifying ways to keep MSCs in the undifferentiated state, with the intention of transplanting them back to repair bone and cartilage [26-31]. Phinney and Prockop argue that mesenchymal stem cells could form the basis for a highly potent “natural system of tissue repair” [32]. Many further experimental models of tissue injuries have demonstrated the therapeutic properties of MSCs upon exogenous administration [33-37]. Prockop pointed out to an interesting fact that in the greater proportion of studies, there has been no correlation between the therapeutic effectiveness and the engraftment efficacy [38]. Furthermore, his findings suggest that the ability to repair was secondary to secretion of soluble factors by MSCs that changed the environment of the tissue [38]. Adult human mesenchymal stem cells have also been shown to have non-immunogenic surface antigens, due to possessing major histocompatibility complex class I protein (MHC I) instead of MHC II [39]. This is undoubtedly a valuable characteristic of adult MSCs which could make it possible to transplant them into an allogenic host without any need for immunosuppression [25]. Many attempts have also been done in order to apply MSCs in gene therapy. This includes their application in Lobstein disease in humans upon systemic administration [40, 41]. Nevertheless, extensive research needs to be done in this field, as our knowledge about their potential use in gene therapy is still very primitive. Also, not much is known about the phenotypic character, developmental origin and their contribution to organogenesis; an assay to demonstrate their self-renewability in vivo is still missing [25].
The therapeutic uses and clinical applications of MSCs have increased research and interest in identifying these cells. Despite 1% of the bone marrow population consisting of hematopoietic cells only 1/10000 to 1/100000 of the bone marrow nuclear cells are MSCs [42, 43]. Moreover, there is not adequate information about the cell surface markers of adult mesenchymal stem cells and how they can be identified. Therefore, correct identification of these cells can be a challenge. There are no articles bringing together and summarizing the cell surface markers of mesenchymal stem cells in adults. The purpose of this study is to summarize all the available information about the cell surface characterization of adult human mesenchymal stem cells by identifying and evaluating all the published literature in this field. The studies reporting mesenchymal stem cell surface markers were searched predominantly using Medline, CINAHL (EBSCO), ZETOC, PubMed, EMBASE and AMED. This systematic review is intended to provide a good basis for identification and selection of adult human mesenchymal stem cells.
MATERIALS AND METHOD
The studies referencing the cell surface markers of adult mesenchymal cells were searched using the electronic databases Medline, CINAHL (EBSCO), ZETOC, PubMed, EMBASE, AMED, PREMEDLINE In-Process & Non-Indexed Citations (OvidSP), ASSIA (CSA Illumina), Conference Proceedings Citation Index: Science (ISI) on Web of Knowledge, PsycINFO (OvidSP), Science Citation Index (ISI) on Web of Knowledge, Social Sciences Citation Index (ISI) on Web of Knowledge and Cochrane Library (Wiley). The following keywords were used to cite relevant articles: adult mesenchymal stem cells, bone marrow-derived multipotent progenitor cells, cell surface profile, MSCs, surface markers. The inclusion criteria were based on 1) characterization of cell surface markers of mesenchymal stem cells 2) identifying mesenchymal stem cells in adults. Studies were excluded which 1) did not comment on the cell surface character of mesenchymal stem cells 2) did not involve mesenchymal stem cells 3) identified mesenchymal stem cells in non adults, or embryo 4) were not available for free viewing. A total of 138 articles were reviewed. 29 articles were identified as relevant according to the inclusion criteria. These studies were summarized and the relevant information is presented in Tables 1-5.
Summary of the Relevant Studies
| Study | Type of Cell in Study | Cell Surface Character |
|---|---|---|
| Dominici et al. [44] | Multipotent mesenchymal stromal cells | These must express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR surface molecules |
| Gronthos et al. [45] | Adult human bone marrow stromal stem cells | Express telomerase activity in vivo |
| Ohgushi et al. [46] | Mesenchymal stem cells | Cells were negative for hematopoietic markers (CD14, CD34, CD45) but positive for markers present in mesenchymal cells CD13, CD29, CD90 |
| Wongchuensoontorn et al. [47] | Mesenchymal stem cells | Found a population of CD34 and CD45 negative cells which were positive for CD44, CD73 and CD105 |
| Gronthos et al. [48] | Human adipose tissue-derived stromal cells | CD9, CD10, CD13, CD29, CD34, CD44, CD 49(d), CD 49(e), CD54, CD55, CD59, CD105, CD106, CD146, and CD166. But no STRO-1 antigen was found as in human adipose tissue derived stromal cells |
| Tsai et al. [19] | Multipotent mesenchymal stem cells | Positive for SH2, SH3, SH4, CD29, CD44 and HLA-ABC (MHC class I), low positive for CD90 and CD105, but negative for CD10, CD11b, CD14, CD34, CD117, HLA-DR, DP, DQ (MHC class II) and EMA |
| Zvaifler et al. [49] | Mesenchymal precursor cells found in the blood | A minority of adult marrow cells express CD34 BMPCs stained strongly with anti-CD44 antibody. Conventional T-cell (CD3), monocyte (CD14, CD68), and B-cell (CD20) antibodies stained neither of the two BMPC populations, nor did they react to anti-LCA (CD45), anti-VCAM (CD106), or MHC-Class II (anti-DR) ADDIN REFMGR.CITE ADDIN EN.CITE.DATA [49] |
| Igura et al. [21] | Placenta-derived mesenchymal progenitor cells (PDMPC) | The PDMPC expressed CD13, CD44, CD73, CD90, CD105 and HLA class I as surface epitopes, but not CD31, CD34, CD45 and HLA-DR. |
| Zuk et al. [50] | Mesenchymal stem cells | Cells expressed CD29, CD44, CD71, CD90, and CD105/SH2 and SH3, expressed STRO-1. No expression of CD31, CD34, and CD45. Positive expression of CD13 and the absence of CD14, CD16, CD56, CD61, CD62(e), CD104 and CD106. CD49(d) was not observed in MSC culture. However MCS expressed CD106 antigen. |
| Miura et al. [51] | Stem cells from human exfoliated deciduous teeth (SHED) | SHED were found to express the cell surface molecules STRO-1 and CD146, which are cell surface markings of adult bone marrow-derived stromal stem cells. |
| Parte et al. [52] | Putative stem cells of menopausal human ovarian surface epithelium | Pluripotent gene transcripts Oct-4, Oct-4A, Nanog, Sox-2, TERT, Stat-3 in human. |
| Iwata et al. [53] | Human bone marrow-derived mesenchymal stem cells | CD29, CD44, CD90 and CD90 |
| Hasebe et al. [54] | Dermal tissue stem cells | Express CD44, CD54, CD90, CD105 and CD271 which are stem cell markers. |
| Yu et al. [55] | Adipose-derived stromal/stem cells | CD29(+) CD34(+), CD44(lo) CD45(low) CD73(+) CD90(+) CD105(+) |
| Kadar et al. [56] | Mesenchymal stem cells | STRO-1 positive |
| Kyurkchiev et al. [57] | Multipotent endometrial stromal cells and multipotent decidual stromal cells | Express surface molecules CD73, CD90 and CD105 |
| Royer-Pokora et al. [58] | Mesenchymal stem cells | CD105, CD90 and CD73. |
| Mousavi et al. [59] | Mesenchymal stem cells | Expression of CD105, CD166 and CD44, and the absence of CD45, CD34 and CD14 on the surface of mesenchymal stem cells like cells |
| Orciani et al. [60] | Mesenchymal stem cells from human skin biopsies (S-MSCs) | Expression of HLA-A, B, C, CD29, CD44, CD73 and CD90, was strongly positive, while CD105 was low positive, and CD10, CD11b, CD14, CD34, CD49d and HLA-DR were negative |
| Bühring et al. [61] | Mesenchymal stem cells in bone marrow | CD13, CD15, CD73, CD140b, CD144, CD146 and CD164 |
| Latif et al. [62] | Mesenchymal stem cells and human cardiac valve ICs (interstitial cells) | Expressed fibroblast surface antigen, smooth muscle alpha-actin, vimentin and CD44. CD105 was weakly expressed by MSCs (> 90%). |
| Gronthos et al. [63] | Bone marrow derived stromal progenitor cells | STRO-1 positive |
| Gronthos et al. [64] | Stromal cells derived from the bone marrow | STRO-1 positive |
| Stewart et al. [65] | Bone marrow-derived mesenchymal stem cells | STRO-1 positive |
| Simmons and Torok-Storb [66] | BM-derived mesenchymal stem cells STRO-1 positive + CD34 positive | STRO-1 positive, CD34 positive |
| Simmons et al. [67] | Bone marrow derived mesenchymal stem cells | CD106 positive, CD34 positive |
| Walsh et al. [68] | Bone marrow-derived mesenchymal stem cells | STRO-1 positive |
| Conget and Minguell [69] | Human bone marrow mesenchymal progenitor cells | Express integrin subunits alpha4, alpha5, beta1, integrins alphavbeta3 and alphavbeta5, ICAM-1, and CD44H. These were CD49d (-) and CD34 (-). |
| Pittenger et al. [6] | Adult human mesenchymal stem cells | CD49d (-), CD106 (+), CD34 (-) |
Summary of Positive Cell Surface Markers of Mesenchymal Stem Cells
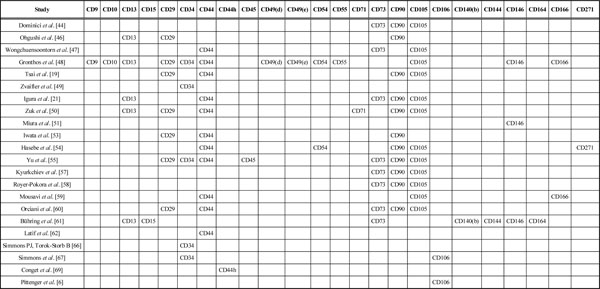 |
Summary of Negative Cell Surface Markers and Mesenchymal Stem Cells
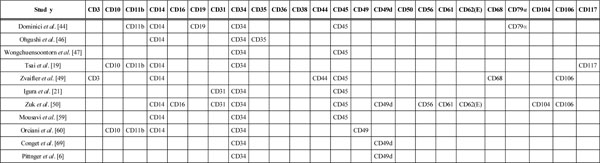 |
The Mesenchymal Cell Positive Surface Markers and the Number of Studies where they have been Reported
| CD105 | 12 |
| CD90 | 11 |
| CD44 | 11 |
| CD73 | 8 |
| CD29 | 7 |
| CD13 | 5 |
| CD34 | 5 |
| CD146 | 3 |
| CD106 | 2 |
| CD54 | 2 |
| CD166 | 2 |
| CD9 | 1 |
| CD10 | 1 |
| CD15 | 1 |
| CD45 | 1 |
| CD49d | 1 |
| CD49e | 1 |
| CD55 | 1 |
| CD71 | 1 |
| CD140b | 1 |
| CD144 | 1 |
| CD164 | 1 |
| CD271 | 1 |
| CD44h | 1 |
The Cell Surface Markers that have been Reported as being Absent on Mesenchymal Stem Cells and the Number of Studies that Reported this
| CD34 | 10 |
| CD14 | 7 |
| CD45 | 6 |
| CD11b | 3 |
| CD49d | 3 |
| CD106 | 2 |
| CD10 | 2 |
| CD31 | 2 |
| CD62e | 1 |
| CD68 | 1 |
| CD117 | 1 |
| CD19 | 1 |
| CD3 | 1 |
| CD16 | 1 |
| CD35 | 1 |
| CD44 | 1 |
| CD49 | 1 |
| CD56 | 1 |
| CD61 | 1 |
| CD104 | 1 |
| CD79α | 1 |
RESULTS
29 studies were identified as relevant according to the inclusion criteria, out of the 138 articles reviewed. Subsequently, these were reviewed carefully and the results summarized in Tables 1 and 2.
As can be seen in Table 1, twenty nine studies looked at mesenchymal cells from different tissues and also identified various cell surface markers characterizing the cells. For easier interpretation, the cell surface markers identified by each study were summarized in Table 2. Gronthos et al. [45] stated that adult human bone marrow stromal stem cells (BMSSCs) or mesenchymal stem cells are non-cyclical and express telomerase activity [45]. Tsai et al. [19] identified the expression of SH2, SH3, SH4 and HLA-ABC (MHC class I) in addition to CD29, CD44, CD90, CD105. They also found that these multipotent mesenchymal stem cells lack expression of HLA-DR, DP, DQ (MHC class II) and EMA. Another study by Gronthos et al. [48] reported that Human adipose tissue-derived stromal cells lack the expression of STRO-1 marker [48]. Positive expression STRO-1 marker was identified by Zuk et al. [50], Miura et al. [51], Kadar et al. [56], Gronthos et al. [63], Gronthos et al. [64], Stewart et al. [65], Simmons et al. [66] and Walsh et al. [68]. Igura et al. [21] found that placenta-derived mesenchymal progenitor cells (PDMPC) express CD105; HLA class I and lack expression of HLA-DR. Zuk et al. [50] reported that mesenchymal stem cells were SH2(+), SH3(+). Parte et al. [52] found putative stem cells of menopausal human ovarian surface epithelium to be positive for pluripotent gene transcripts Oct-4, Oct-4A, Nanog, Sox-2, TERT, Stat-3. Yu et al. [55] reported that there is a low expression of CD45 and CD44 in Adipose-derived stromal/stem cells (ASC). Orciani et al. [60] identified the MSCs from human skin biopsies (S-MSCs) to be positive for HLA-A, B, C, and low positive for 105 and negative for HLA-DR. Latif et al. [62] reported mesenchymal stem cells to be positive for fibroblast surface antigen, smooth muscle alpha-actin, vimentin and low positive for CD105. Zvaifler et al. [49] found that only a minority of bone marrow cells expressed CD34. Conget and Minguell [69] reported the expression of integrin subunits alpha4, alpha5, beta1, integrins alphavbeta3 and alphavbeta5, ICAM-1, and CD44H in human bone marrow mesenchymal progenitor cells.
DISCUSSION
In this systematic review we have looked at the cell surface characterization of adult mesenchymal stem cells. We have found that CD105, CD90, CD44, CD73, CD29, CD13, CD34, CD146, CD106, CD54 and CD166 rank among the most commonly reported positive cell surface markers on mesenchymal cells. There are, however, a number of cell surface markers that have been reported as being absent in mesenchymal stem cells. The most frequently reported are CD34, CD14, CD45, CD11b, CD49d, CD106, CD10 and CD31. In addition to these, a number of other cell surface markers have been identified. These are STRO-1, SH2, SH3, SH4, HLA-A, HLA-B, HLA-C, HLA-DR, HLA-I, DP, EMA, DQ (MHC Class II), CDIO5, Oct 4, Oct 4A, Nanog, Sox-2, TERT, Stat-3, fibroblast surface antigen, smooth muscle alpha-actin, vimentin, integrin subunits alpha4, alpha5, beta1, integrins alphavbeta3 and alphavbeta5 and ICAM-1 [19, 21, 50, 50-52, 56, 60, 62-66, 68, 69]. Eight studies have confirmed the expression of STRO-1on bone marrow-derived mesenchymal stem cells [50, 51, 56, 63-66, 68]. However, Gronthos et al. [48] has reported adipose tissue-derived stromal cells to be STRO-1 negative. This suggests the difference in the expression of STRO-1 depending on whether they are adipose tissue-derived or bone marrow derived; nevertheless it should be noted that these represent only about 3% of the bone marrow stromal population [48, 63-68]. Positive expression of SH2 and SH3 has been confirmed by Tsai et al. [19] and Zuk et al. [50]. Igura et al. [21] Tsai et al. [19] and Orciani et al. [60] have all confirmed that mesenchymal stem cells are HLA-DR negative. Orciani et al. [60] and Latif et al. [62] have both stated that there was low expression of CD105. Another interesting finding in this systematic review is that several studies have reported conflicting findings about the cell surface markings of mesenchymal stem cells. These cells were identified as CD10 (+) by Gronthos et al. [45], but as CD10 (-) by Tsai et al. [19] and Orciani et al. [60]. Similarly Gronthos et al. [45], Zvaifler et al. [49] and Yu et al. [55] identified the cells as CD34 (+) whereas Dominici et al. [44], Ohgushi et al. [46], Wongchuensoontorn et al. [47], Tsai et al. [19], Igura et al. [21], Zuk et al. [50], Mousavi et al. [59] and Orciani et al. [60] found these to be CD34 (-).
In a study to identify the mesenchymal precursor cells in the blood of normal individuals, Zvaifler and colleagues reported on interesting observations. They found that only a minority of these cells express the CD34 surface antigen [49]. They also reported that only 5% of marrow cells reacted with Stro-1 antigen which is one of the defining features of bone marrow MSCs [49, 66]. Moreover, Zvaifler et al. stated that the colony forming unit fibroblasts expressed much lower levels of CD34 compared to human hematopoietic stem cells [49]. Other studies have found that CD34 is also expressed on vascular endothelial cells, basement membrane structures, and dendritic and perifollicular cells in human skin [70, 71]. Simmons and Torok-Storb used the expression of CD34 marker to separate human bone marrow cells [72]. As suggested by Zvaifler et al. the difference in findings concerning the CD34 antigen could be because all the studies mentioned above were conducted on MSCs before the culture [49]. The same stromal cells after culture, in vivo, do not react with CD34 antibodies anymore [72]. Similarly, despite having been identified in the endothelial cells of human umbilical vein, they have not been found in vitro [73]. According to these results we can conclude that the CD34 expression of these cells is highly disputed [74, 75].
CD44 is another cell surface marker that has been identified as positive [19, 21, 45, 47, 50, 53-55, 59, 60, 62] and negative [49]. Yu et al. [55] stated that adult mesenchymal stem cells are CD45 positive as opposed to findings by Dominici et al. [44], Wongchuensoontorn et al. [47], Zvaifler et al. [49], Igura et al. [21], Zuk et al. [50] and Mousavi et al. [59] where they have been reported as CD45 negative.
It is important to note that Yu et al. [55] reported adult MSCs to express low levels of CD44 and CD45. Gronthos et al. [45] found the cells to be CD49d positive, whereas Zuk et al. [50] reported them to be CD49d negative. Finally, Simmons et al. [67] and Pittenger et al. [6] have identified mesenchymal stem cells as CD106 positive but, Zvaifler et al. [49] and Zuk et al. [50] have found them to be CD106 negative.
A possible explanation for the conflicting results obtained in different papers reviewed is provided in a study conducted by Gronthos and colleagues where gene and protein expression of human adipose tissue-derived stromal cells in their differentiated and undifferentiated states is described. The results obtained were then compared to those of bone marrow stromal cells as previously defined in the literature. As already mentioned in this paper, they found that the expression of surface antigen in adipose tissue-derived stromal cells is not identical to that of bone marrow-derived stromal cells [48]. In this study, two different explanations for the discrepancy in the results have been suggested. Firstly, they argue that these differences may be due to different proliferative stages of the cells in culture [48]. Furthermore, they also state that donor heterogeneity could be of importance when comparing adipose tissue-derived stromal cells with those derived from the bone marrow [48].
In this systematic review we have managed to look at many different studies which discuss the different cell surface markers of adult MSCs. According to the literature reviewed, we can say that there is great discrepancy between the existence and expression of many cell surface markers including CD10, CD34, CD44, CD45, CD49d and CD106, which at times makes it difficult to summarize the available evidence in order to establish a set of clear-cut criteria for identifying these cells. As mentioned earlier, there may be different reasons as to why the expression of these surface markers differs in a number of studies. However, we suggest that further research is needed to identify the cell surface profile of adult MSCs. One possibility would be to choose adult MSCs of the same origin, say bone marrow-derived MSCs, and compare their surface characteristics under the same conditions as other reviews have already provided. Subsequently, the characteristics of a group of cells of the same origin should be assessed under different conditions, such as before and after culture, to observe whether there is any change in the expression of surface markers.
While we hope to have established a good reference for identifying the cell surface markers on adult mesenchymal stem cells, it is important to point out some limitations of this systematic review. 1) The reported cell surface characteristics in the studies reviewed were not all derived from the same tissue. Some were bone marrow-derived [45; 50; 51; 53; 56; 61; 63-66; 68], some from skin [54; 60] and others from endometrium [57], adipose tissue [48; 55], ovaries [52], deciduous teeth [51] and many other sources. 2) Some of the studies included were not specifically aimed at identifying the cell surface markers of adult mesenchymal cells. These studies simply included the surface markings that were identified or compared to other cells. 3) There were fewer studies looking at the negative surface markers of mesenchymal stem cells compared to those identifying the positive cell surface markers. 4) Despite the fact that there are not plenty of studies commenting on identifying adult mesenchymal stem cells, we could argue that the number of studies reviewed is not very large and thus the results need to be interpreted with care.
CONCLUSION
In this systematic review we have summarized the available research evidence concerning the cell surface profile of adult mesenchymal stem cells. The findings have been presented in Tables 1-5. Different studies have identified various positive and negative surface markers for mesenchymal stem cells. Interestingly, several studies have reported conflicting information about some of the cell surface markers including CD10, CD34, CD44, CD45, CD49d and CD106. We can conclude that the expression of some surface antigens, such as STRO-1, is dependent on whether the cells are adipose tissue-derived or bone marrow-derived. Another factor accounting for the variability in the expression of adult MSC surface markers could be the different stages during cell proliferation and culture where the markers have been accessed. Despite the exhaustive findings of this systematic review, the conflicting evidence and inadequate information about several cell surface markers, we suggest that further research in this field is necessary.
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGEMENT
None declared.


