All published articles of this journal are available on ScienceDirect.
Compartment Syndrome Following Lower Limb Arthroplasty: A Review
Abstract
Compartment syndrome is an urgent clinical entity characterised by an increase in the interstitial pressure within a closed osseofascial compartment. Although well recognised as a potential complication after orthopaedic trauma, it is very rarely presented after elective orthopaedic surgery and especially joint arthroplasty. In these rare cases a number of variables are associated with it (positioning, coagulopathy, extensive soft tissue dissection, previous scarring, and epidural analgesia). In this study we present the current evidence with regard to incidence and causation of compartment syndrome after lower limb joint arthroplasty and make recommendations on how to avoid the development of this devastating complication.
INTRODUCTION
Acute compartment syndrome (CS) of the lower extremity is a surgical emergency induced by bleeding, or oedema within a closed, non-elastic muscle compartment, leading to high intra-compartmental pressures compromising local tissue perfusion [1]. Prompt diagnosis is of paramount importance in order to avoid irreversible tissue changes leading to muscle necrosis, neurological deficit, myoglobinuria, renal failure, and potentially mortality.
The most common cause of CS is trauma, usually orthopaedic or vascular [2-4]. Other aetiologies that have been associated with the development of a CS, include exercise [5], positioning during prolonged surgical procedures [6], intramedullary nailing [7], prolonged tourniquet application [8], anticoagulation [9], and intravenous drug abuse [10]. CS has also been reported after elective orthopaedic procedures, mainly concerning total hip and knee arthroplasty. Following the gained popularity of lower limb arthroplasty [11], and the absence of an analysis of the resulting CS, the herein study aims to investigate the existing evidence on this complication in an effort to identify causative factors, and propose an algorithm of prevention and management.
MATERIALS AND METHODOLOGY
An electronic search of the MEDLINE database (up to December 2009) was carried out entering the terms and Boolean operators: “compartment syndrome” AND “arthroplasty”. It has resulted to 29 studies. In addition, we reviewed the references from the resulting publications to identify further potential articles that could be included to our study. Review papers and editorials were excluded (Table 1). All articles were screened with set inclusion and exclusion criteria.
A Flowchart Illustrates our Study Selection Process
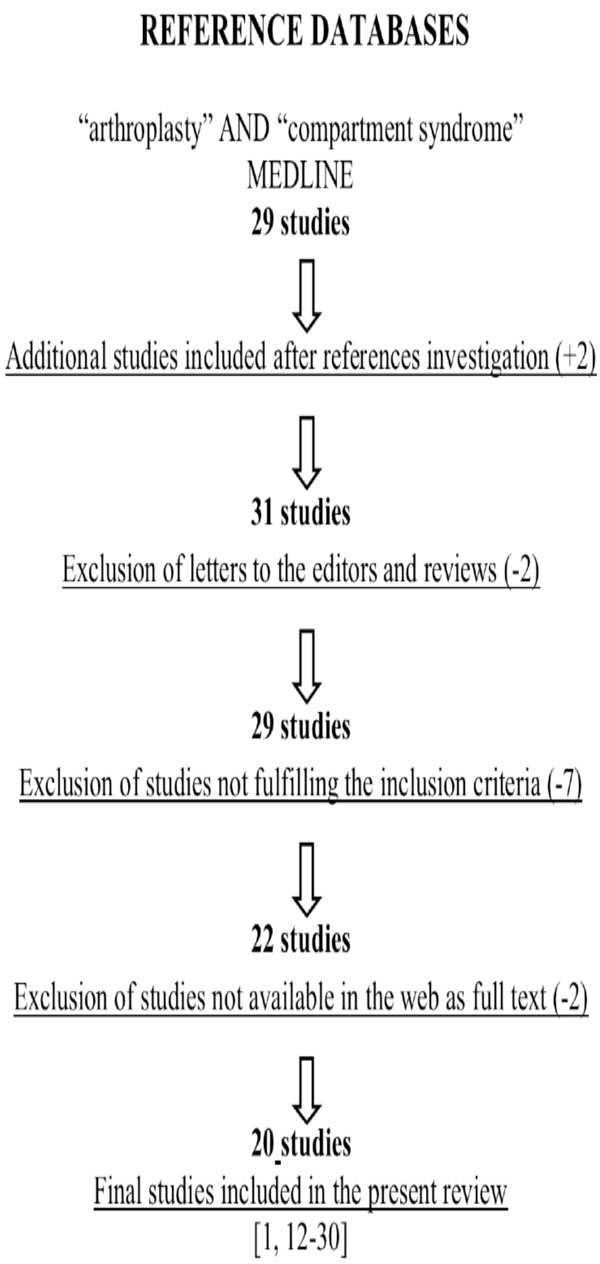 |
Articles were considered eligible if they met the following inclusion criteria: (1) a lower limb arthroplasty procedure should be described; (2) the symptoms or the complications described should correspond to a compartment syndrome. Exclusion criteria included studies that examined arthroplasty treatment in the setting of acute trauma, review articles, editorials, letters, and manuscripts in other than the English language.
Two separate reviewers (NGL, NKK) were involved in the screening process, and classified the retrieved studies independently, either as relevant or non-relevant to the present review. Any disagreements were resolved between the 2 reviewers after reading the manuscript’s abstract.
Selected studies were original articles either in the form of case reports, or case series of lower limb joint arthroplasty in which CS was recorded between the complications. All relevant information regarding patients’ age and gender, type of arthroplasty, reason for surgery, anatomical body region and involved compartments, positioning, use of tourniquet, bleeding diathesis, thromboprophylaxis administration, type of post-operative analgesia, operating time, time to discontinuation of epidural effusion, symptoms initiation and fasciotomy, neurovascular deficit, intracompartmental pressure (ICP) reading and complications were all recorded (Table 2).
Description of the Parameters of the Reviewed Studies
 |
RESULTS
In total twenty manuscripts met the eligibility criteria dating from 1979 to 2007 [1, 12-30] (Table 1). Thirteen studies consisted of a single case report [12, 13, 15, 16, 18-22, 24-27], while 7 studies included small series of patients (2-7) either undergoing the same type of arthroplasty or developing a CS on the same site [1, 14, 17, 23, 28-30]. The first three chronological studies [28-30] did not specifically refer to the typical sequel of a CS; nonetheless, since the main parameters examined in those studies (myoglobinuria, nerve paralysis) were strongly related to CS pathophysiology, it was decided that they should be included.
Forty-one cases were described in total, including 22 after arthroplasty of the hip [14, 16, 18, 20, 23, 25-30], 18 of the knee [1, 12-15, 17, 21-24], and 1 of the forefoot [19]. Five different anatomical areas were involved including gluteal [14, 16, 17, 26, 28-30], thigh [1, 20, 23, 24, 27], calf [1, 12, 13, 15, 21, 22, 25], forefoot [19], and forearm compartments [18]. Twenty-five patients were males and 15 females with an overall average of 54.8 years of age (range 37-75). Degenerative arthritis was the reason for arthroplasty in the majority of cases (23/40) followed by loosening of implants (8/40), Avascular Necrosis (AVN) (3/40) and other reasons.
Of the 22 patients that underwent total hip arthroplasty (THA), 16 (72.7%) developed CS of the gluteal compartment (buttocks), 4 (18.2%) of the thigh, and one (4.5%) of the calf. From the 18 patients that underwent a total knee arthroplasty (TKA), 4 (22.2%) developed a CS in the gluteal area, 3 (16.6&) in the thigh, and 11 (61.1%) in the calf compartments (Table 3). A forefoot CS was recorded after revision forefoot arthroplasty, as well as an arm CS after a revision THA.
Number of Cases in Terms of Localisation of Arthroplasty, Localisation of Compartment Syndrome (CS) & Potential Reasons
| Arthroplasty Joint | Gluteal CS | Thigh CS | Calf CS | |
|---|---|---|---|---|
| Total no of cases | Hip | 17 | 4 | 1 |
| Knee | 4 | 3 | 11 | |
| Positioning | Hip | 14 | — | — |
| Knee | 2 | — | — | |
| Routine thromboprofylaxis | Hip | 1 | 4 | 1 |
| Knee | — | 2 | 2 | |
| Bleeding predisposition | Hip | 1 | 3 | |
| Knee | 1 | 1 | 1 | |
| Aggressive Physiotherapy | Hip | — | — | — |
| Knee | 1 | 1 | — | |
| Extensive soft tissue dissection | Hip | 7 | 2 | |
| Knee | — | — | 3 | |
| Previous operations on site | Hip | 8 | — | — |
| Knee | — | — | — | |
| Continuous Epidural anaesthesia | Hip | 3 | — | 1 |
| Knee | 4 | — | 9 |
The mean operating time was 196 min (65 – 420), and the mean time from surgery until the CS diagnosis was 26 hours (0 – 72) [1, 13-18, 21, 27, 29]. The mean time to fasciotomy was 53.2 hours (8 – 144) [1, 12-21, 23, 25, 26, 28, 30]. In the cases that epidural analgesia was used, the mean time to its discontinuation was 30.8 hours (12 – 48) [1, 12, 14-17, 21]. Neurovascular parameters were recorded in some of the studies identifying absence of peripheral pulses just 13% (4/31) of all cases [1, 13, 15-21, 23, 24, 28-30], and nerve deficit (sensory or motor) in 68.42% (26/38) [1, 13-20, 23-30]. Intracompartmental pressure (ICP) measurement data were recorded in 6 of the 20 studies (8 cases) [1, 20, 21, 23-25, 28] with readings ranging from 32 to 110 mm Hg. In two other reports [15, 18] ICP measurement was not conducted, as it was considered unnecessary, due to the presence of clear clinical signs. In all cases where the ICP was recorded, it was found to be elevated leading to subsequent fasciotomies. Full recovery without any secondary sequel of CS was recorded in 52.63% of the cases (20/38) [1, 14, 18-20, 23, 24, 26, 28-30]. In contrast, 36.8% (14/38) developed motor deficits (most usually a drop foot) [1, 13, 14, 16, 17, 21, 23, 27, 28], and 26.3% (10/38) of the patients a sensory deficit (numbness, paresthesias) [12, 13, 17, 21, 23, 27, 28, 30]. One patient eventually [1] had a below the knee amputation because of irreversible equinus deformity and foot drop (Table 2).
The aetiology of CS was not clearly defined in all cases and mostly retrospective speculative assumptions were recorded. Prolonged positioning of the patients either intra-operatively (lateral decubitus) [18, 25, 28, 29], or during the nursing phase (supine, semirecumbent) [14, 17] was considered exclusively responsible for the gluteal CS after THA or TKA. Bleeding predisposition either related to long term drug administration (warfarin [20, 23]/aspirin [14]), or routine thromboprofylaxis (low molecular weight heparin subcutaneously [13, 20, 23, 25], oral warfarin [24], or intravenous heparin [30]) was mainly related to CS of the thigh [20, 23, 24, 27], or buttock [14, 30]. Out of 26 cases that recorded the type of post-operative analgesia, continuous epidural infusion was used in 17, and was considered as responsible for a delayed CS diagnosis in 5 of them [13-16, 21]. A tourniquet was used in 16 cases (at a level not higher than 350mmHg) of TKA, or unicompartmental knee arthroplasty (UKA) and in 1 case of forefoot arthroplasty. Its use was considered responsible for the consequent CS for only 1 patient [15].
Obesity for 9 patients [14, 29], vascular thrombosis compression or rupture for 9 patients [1, 20, 21, 25, 26, 30], extensive soft tissue dissection for 6 patients [1, 20, 23, 26], scarring due to previous operations for 4 patients [1, 19], vigorous early physiotherapy for 2 patients [17, 24] and intra-operative use of calf stimulators for 1 patient [25] were reported as causative factors for CS after elective lower limb joint arthroplasty.
DISCUSSION
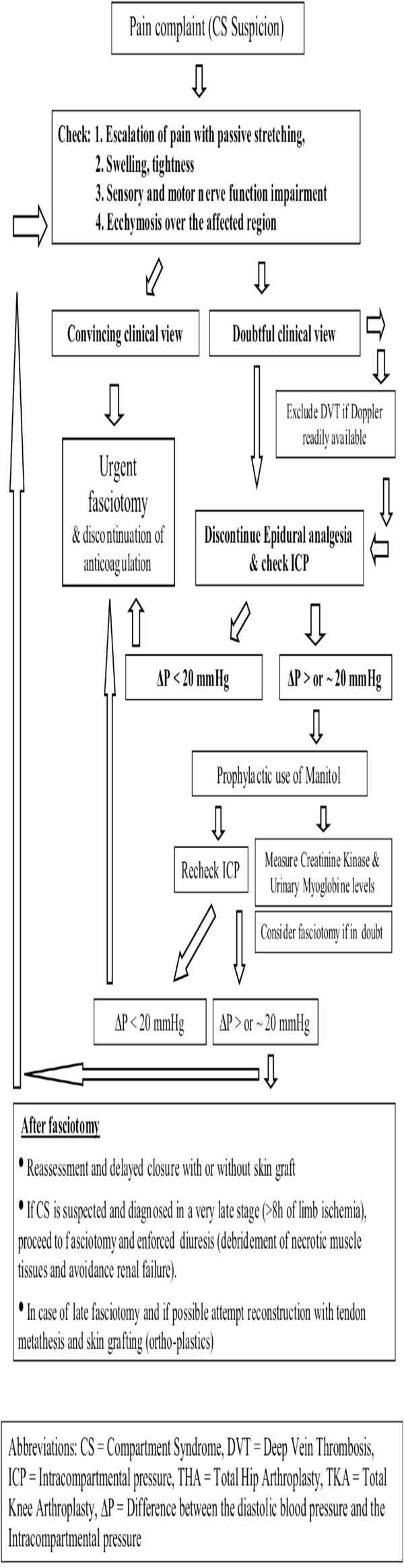
The pathophysiology of compartment syndrome is characterised by a unique type of ischemia that affects a group of muscles enclosed within a relatively non-expandable fascial sheath and bony structures [31]. Its early signs include severe pain disproportionate to the injury, exacerbated by passive stretching of the involved muscles. Paresthesias may occur at an early or later stage. Swelling, coldness, and paralysis follow if surgical intervention is postponed [1]. Pulseness may or may not occur, thus the presence of a peripheral pulse does not rule out the presence of CS [32]. Clinical symptoms are usually adequate to make the diagnosis. In doubtful cases, or in unconscious patients, intra-compartmental pressure measurements are helpful to set the diagnosis and to proceed with the subsequent urgent fasciotomies. Missed diagnosis and late decompression is associated with significant morbidity due to irreversible ischemic necrosis of the muscles and nerves within the compartment [33]. In neglected cases severe systemic complications may follow (myoglobinuria and acute renal failure), or even death [28].
One of the main drawbacks is that the timing of the onset of CS cannot always be precisely defined. Moreover, when CS occurs after “low risk” operations, such as joint arthroplasty, the chance of remaining undiagnosed the first valuable hours is high. This delay, may lead to dramatic consequences secondary to irreversible ischemia of the nerves and muscle tissue [34].
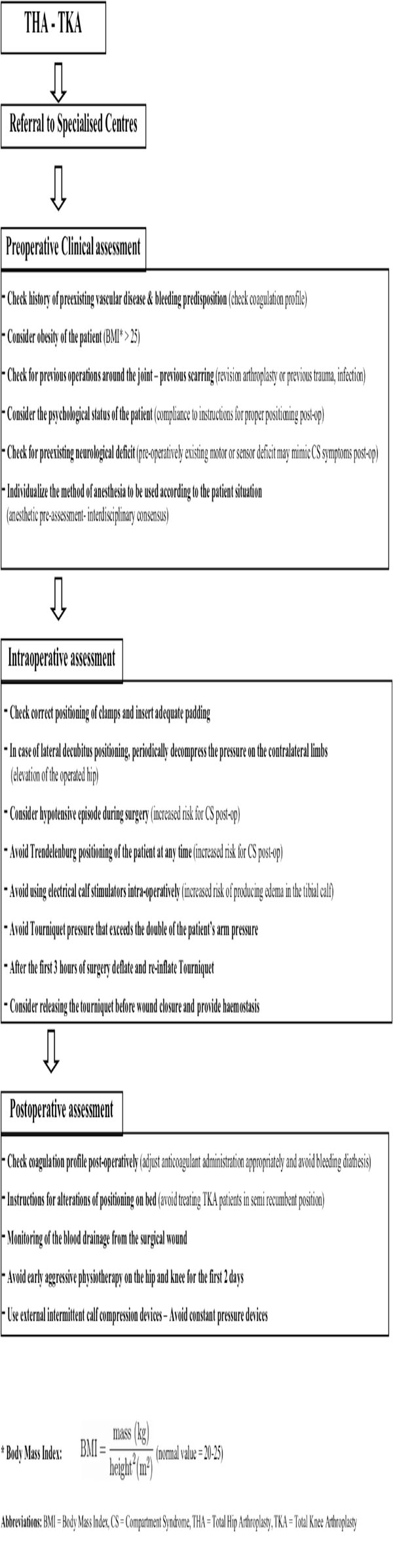
Our study aimed to identify and comprehensively present the existing evidence on CS aetiology related to elective joint arthroplasty of the lower limb. Despite the small number of cases which were retrieved from the published series in PubMed, useful suggestions related to CS prevention and management could be made. Firstly, it is of paramount importance that all the staff (medical and paramedical) involved in the perioperative care of lower limb arthroplasty patients is aware of the clinical presentation and importance of early diagnosis and management of compartment syndrome. Secondly, specific precautionary measures need to be applied as routine practice:
- Positioning: It was evident from the reviewed studies that the lateral decubitus positioning during THA surgery was highly correlated with gluteal CS. This was mainly attributed to the incorrect placement of the posterior pad causing compression on the gluteal muscles [28], or to the firm placement of the anterior clamp that pushes the contralateral thigh and occlude the femoral neurovascular bundle causing sensory and motor deficits [29]. It is characteristic that in almost all of the THA – gluteal CS cases [14, 16, 28, 29] the syndrome was developed in the contralateral buttock incriminating positioning as the causative factor, and indicating the need for careful placement of the posterior and anterior clamps, in order to avoid compression of the gluteal muscles or of the femoral triangle. Adequate padding between the clamps and the body of the patient should be applied. There were also 2 studies [14, 17] in which post TKA gluteal CS was attributed to postoperative nursing in a semi-recumbent position. The clear message from these 2 studies was that vigilant nursing care for regular change of posture and for recognition of motor blockade is mandatory especially for obese patients under epidural analgesia. Considering CS related to patient’s positioning, surgeons should always keep in mind the higher risk due to the Trendelenburg inclination. This is usually associated with concomitant lithotomy position of the legs [35] which is not used in lower limb joint arthroplasty. Nonetheless in the setting of hypotensive anaesthesia, which is by itself a predisposing factor for diminished blood perfusion, the addition of prolonged Trendelenburg inclination may create unwanted consequences and should be avoided.
- Tourniquet use: Pneumatic tourniquets maintain a relatively bloodless field during knee or forefoot arthroplasty, aid identification of vital structures, and expedite the procedure [36]. However, after prolonged use they may induce an ischemia-reperfusion injury causing CS [8, 36]. Safe limits of duration under tourniquet and levels of pressure are debatable. Worland [37] stated that the tourniquet pressure should be limited to 100 mmHg above systolic pressure, while Klenerman and Hulands [38] describe the ideal tourniquet pressure for the thigh to be double that of the systolic pressure in the arm. Moreover, they proposed the time safety limit of three hours for continuous tourniquet use. In the reviewed studies that reported CS after TKA the authors did not correlate its incidence to the tourniquet use. A possible explanation may be that the recorded tourniquet times and pressures were all well within the established safety limits.
- Calf compression devices: Calf compression devices have been implicated in the past for the development of compartment syndrome [39]. In one of the studies of this review [25] intraoperative use of calf stimulation devices was considered a potential reason for CS. In contrary, later evidence [40] advocated that external intermittent compression devices decrease compartment pressures by improving venal return in contrast to antithrombotic elastic stockings. Use of interchangeable calf compression devices is recommended, however their contribution in the avoidance of compartment syndrome needs to be investigated further.
- Physiotherapy: Post-operative physiotherapy should avoid early aggressive passive flexion of the hip or the knee. In patients post-TKA that commence early continuous passive motion, flexion to 30° was found to produce dramatic pressure elevation up to 35 mmHg creating CS in the thigh or the gluteal compartment [17, 24]. It may be preferable to restrict joint mobilisation protocols to active-assisted passive or simple active flexion-extension exercises for the first 2 days post-operatively. More aggressive apparatus assisted physiotherapy could follow since the patient would have indiscriminately overcome the first stage of mobilisation.
- Post-op analgesia: Increasing pain despite analgesic medication is the main CS symptom and should always alert clinicians. Deep sensory and motor epidural blockade excludes pain as a constant indicator for compartment syndrome since it is inevitably no longer present. This, in combination with the low index of suspicion for CS may create a disastrous combination. It is essential, when using local epidural anaesthetics that the intensity of the block is appropriate to the anticipated intensity of the wound pain without inducing motor blockade [41]. Nonetheless, analgesia should not be misunderstood as the cause but only as being a factor delaying the diagnosis. According to a recent review [42] there was no convincing evidence that epidural analgesia or patient-controlled analgesia delays the diagnosis of compartment syndrome provided patients are adequately monitored. This matches with the findings of our review since continuous epidural analgesia was considered responsible for totally masking the symptoms in only 2, poorly monitored, patients out of 17 that received it. Thus, it can be suggested that whatever the mode of analgesia used, a high index of clinical suspicion, ongoing assessment of patients, and compartment pressure measurement are of outmost importance for an early diagnosis [42].
- Anticoagulant administration: Pharmacological prophylaxis against venous thromboembolism may be associated with serious haemorrhagic risks [43]. Bleeding predisposition in patients receiving anticoagulants on a permanent base due to vascular disease should be taken into account. Those patients need meticulous monitoring throughout the perioperative period. For patients not chronically receiving anticoagulants the type and the dose of prophylaxis remains unclear with certain differences existing between American and European protocols [43]. In some of the reviewed studies the use of anticoagulants was considered as a causative factor [23, 27, 30]. Nonetheless, the correlation could not be clearly proved, and moreover other reasons, such as extensive soft tissue dissection [23] or general hypoxia of the limb [30], coexisted. Patients undergoing joint arthroplasty should receive routine anticoagulation therapy, based on the local protocols of every institute. In any case though, the coagulation profile (fibrinogen, FDP, platelets, INR) needs to be evaluated pre- and post-operatively and specialist consultation requested if necessary. As soon as a suspicion related to CS is raised, anticoagulation needs to be halted and its effect reversed if possible.
- High risk patients: In those cases where prolonged time of operation, hypotension during anaesthesia, lateral decubitus positioning, obesity, previous operations on the same joint, vascular disease are present, meticulous clinical monitoring, and if feasible, measurement of the compartment pressures, should become the standard of practice [44]. Measurement of the intracompartmental pressure (ICP) is important, but should not replace clinical judgement [8, 45]. The diagnosis of CS is mainly clinical, however it should not be forgotten that pain may be an unreliable symptom as it is subjective and variable [42]. It may be absent in established acute compartment syndrome associated with nerve injury, or minimal in deep posterior compartment syndrome [44]. Thus, ICP measurement is useful in doubtful cases as well as in cases in which epidural analgesia may mask the symptoms. Furthermore, it is useful in identifying which of the compartments are involved. Decompression is generally recommended when the compartment pressure exceeds 30 mmHg to 35 mmHg [8, 41, 44]. The view that fasciotomy should be generally performed when tissue pressure rises over 20 mmHg below diastolic pressure [34, 46] appears to be of wide acceptance lately. Deep vein thrombosis (DVT) may also be present causing thigh swelling and firmness on palpation. It should be excluded before proceeding to fasciotomy [47]. It has been stated that muscles tolerate well 4 hours of ischemia, by 6 hours the result is uncertain, and after 8 hours the damage is irreversible [34]. Even if these values are indicative and may vary depending on the clinical setting or the patient’s profile, time is precious. The need for early fasciotomy is absolute and decompression of all the affected anatomical compartments is recommended. It should be stated however, that fasciotomies are not hazardless procedures, and there is some evidence that they may cause chronic venous insufficiency due to impairment of the calf muscle pump, gross scars, and increase infection rates [48].
- Late Decompression: The role of fasciotomy in cases of compartment syndrome which has been diagnosed at a late stage (after 8 hours) is questionable. Established myoneural deficits seldom recover after late fasciotomies [49]. Furthermore, fasciotomies performed after 35 hours from injury were invariably associated with severe infection and even death [49]. Nonetheless, even if a compartment syndrome is suspected at a stage when fasciotomy may be too late, the salvation of the compartment should be attempted. The necrotic contents of the compartment need to be debrided, to avoid myoglobinuria associated complications, while early tendon metathesis combined with skin grafting may provide a reasonable functional result.
The CK values are considered as a sensitive marker of ongoing rhabdomyolysis. CK values of over 2,000 U/l after surgery may be considered a warning sign in ventilated and sedated patients, in whom early clinical symptoms of the compartment syndrome such as pain and paresthesias cannot be assessed [50]. The presence of myoglobinuria suggests an impending CS but the amount of myoglobinuria does not correlate with the degree of underlying tissue injury [51]. Prophylactic use of mannitol may obviate the need for decompressive fasciotomy [41], however if myoglobinuria is established enforced diuresis is recommended in order to avoid renal complications [50].
The importance of prevention and early diagnosis of the compartment syndrome is paramount for those patients at risk. Surgeons and nursing staff need to be familiar with this complication and follow strategies for its prevention (Tables 4 and 5). The rarity of CS following joint arthroplasty, and the difficulty of obtaining large prospective series limits the strength of the existing evidence. As it was previously reported [6], elective and reconstructive procedures, such as joint arthroplasty, should be performed in specialist centres where the use of specific preventive and monitoring protocols are optimal.
Perioperative Assessment for Prevention of Joint Arthroplasty Related Compartment Syndrome for Conscious Patients
 |
Algorithm for Management of CS After THA or TKA
 |
CONFLICT OF INTEREST STATEMENT
The authors would like to confirm that there were not any financial or personal relationships with other people or organisations that could inappropriately influence (bias) the material and the results of this manuscript.


