All published articles of this journal are available on ScienceDirect.
Significance of LRP and PPAR-γ Expression in Lipomatous Soft Tissue Tumors
Abstract
Background:
Molecular mechanism of differentiation in lipogenic tumor is still unknown in detail. Low-density lipoprotein receptor-related protein (LRP) and peroxisome proliferator-activated receptor gamma (PPAR-γ), representative regulatory molecules of lipogenic differentiation, have been reported today as multi-functional molecules and to modulate tumorigenesis in various kind of cancer. To date, diagnostic and therapeutic significance of the expression of these molecules in lipogenic tumors are not defined.
Methods:
The immunohistochemical expression status of LRP and PPAR-γ in various grades of 54 lipogenic tumors was analyzed. Correlation between the expression levels and the differentiation of the tumors was confirmed. For statistical analyses, the Kruskal-Wallis test, the Steel-Dwass test and the Mann–Whitney U test were used.
Results:
LRP and PPAR-γ expression was detected in 50 (92.6%) and 44 (81.5%) cases, respectively. The expression level in LRP was significantly higher in cases with well differentiated liposarcoma, pleomorphic liposarcoma and dedifferentiated liposarcoma than in lipoma. Compared with lipoma or well differentiated liposarcoma, significant elevation in expression level of PPAR-γ was confirmed in myxoid liposarcoma, pleomorphic liposarcoma, dedifferentiated liposarcoma and the differentiated area of dedifferentiated liposarcoma.
Conclusion:
The up-regulation of LRP and PPAR-γ in higher grade cases, i.e. less differentiated tumors than in low grade cases was shown, suggesting the candidate role of these molecules as tumor progression modulators rather than regulatory molecules of differentiation in lipogenic tumors.
INTRODUCTION
Low-density lipoprotein receptor-related protein (LRP) is a cell surface receptor that is frequently found in the liver, placenta, brain, epithelial cells of the digestive system, smooth muscle cells, macrophages and fibroblasts [1-3]. LRP is a member of the low-density lipoprotein receptor (LDLR) family, which comprises LRP, LDLR, very low-density lipoprotein receptor and other several molecules [4]. When LRP was originally identified, the structural similarities to LDLR and expression in the liver suggested a role in lipoprotein metabolism and cholesterol homeostasis. Further in vitro evidence that LRP binds apolipoprotein E (apo E) led to the proposal that this molecule serves as a receptor for chylomicron remnants, lipoproteins that primarily shuttle dietary cholesterol from the gut to the liver. However, considerable evidence began to emerge suggesting that a major function of LRP lies in the removal of proteinase and proteinase inhibitor complexes, raising the possibility that this molecule acts as a multifunctional scavenger receptor [4]. To date, LRP has been reported to bind and endocytose over 30 structurally and functionally distinct ligands, including proteinases such as matrix metalloproteinases (MMPs) and urokinase-type plasminogen activator (uPA) [4]. The importance of these proteolytic enzymes in tumor progression suggests a critical role for this molecule in malignancy. In fact, LRP expression has been reported in various kinds of tumors, including astrocytoma, colon cancer, renal cancer and melanocytic tumor [5-8]. However, the precise roles and potential underlying mechanisms for this molecule in malignancy remain controversial.
Peroxisome proliferator-activated receptors (PPARs) are members of a super family of nuclear receptors. The PPAR subfamily has three isotypes: α, β/δ, and γ, which are ligand-activated transcription regulators that are important in cellular homeostasis [9]. Among these, PPAR-γ is a nuclear hormone receptor that plays a critical role in adipocyte differentiation and control of lipid uptake [10]. The molecule is expressed in preadipocytes at limited levels and is turned on during differentiation, prior to the expression of most adipocyte genes, many of which contain PPAR-binding sites [11]. Expression of this molecule in malignancy, induction of differentiation by ligands of this molecule in cancer cells, and growth inhibition of tumor by specific ligands all imply roles for PPAR-γ in tumorigenesis [12-14]. A recent in vitro study of a liposarcoma cell line found that expression levels of LRP were regulated by PPAR-γ [15].
Common properties of these two molecules such as close relationships with tumorigenesis and regulatory effects on fat metabolism or lipogenic differentiation strongly suggest that these molecules probably act as key players of regulation in the cell function of lipogenic tumors. However, limited data has been available regarding the expression status of these molecules in lipogenic tumors [10, 16]. Liposarcoma is one of the most frequent soft tissue sarcomas in adults, and has usually been divided into four major categories classically based on histological findings: well-differentiated liposarcoma (WDLS); dedifferentiated liposarcoma (DDLS); myxoid/round cell liposarcoma (M/RLS); and pleomorphic liposarcoma (PLLS). As with other numerous malignancies, intensity of differentiation is believed to regulate histological grade and prognosis. Recent fruits of genetic analysis depict the profound molecular mechanisms affecting morphology in lipomatous tumors. Recent reports have shown close relationships between WDLS and 12q13-15 region amplification, between DDLS and 1p32 or 6q23 amplification, and between M/RLS and the t(12;16) translocation [17, 18]. Despite these intensive analyses, details of molecular events in the common regulatory mechanism of lipogenic differentiation are still unknown. We examined herein the expression of LRP and PPAR-γ in lipogenic tumors, including lipoma and various grades of liposarcoma in order to seek the fundamental, diagnostic and therapeutic significance of the expression of these two molecules under the hypothesis that these molecules play a critical role in the regulation of lipogenic tumor malignancy and differentiation.
MATERIALS AND METHODS
Specimens were obtained from 54 patients who had undergone surgical resection or open biopsy at the registered institutes. Histological diagnosis was confirmed by standard light-microscopic evaluation of sections stained with hematoxylin and eosin by two authors (Y.F. and F.K.). Classification of tumors used in this study was based on the revised World Hearth Organization (WHO) criteria for soft tissue tumors with minor modification [19]. Tumors that did not contain round cell components throughout the specimen were independently myxoid liposarcoma (MLS) among the tumors diagnosed as M/RLS. Cases that needed subtle differential diagnosis such as lipoma and WDLS were subjected to chromosomal analysis. Finally, specimens were diagnosed as lipoma in 15 cases, WDLS in 12 cases, MLS in 11 cases, M/RLS in 5 cases, PLLS in 6 cases and DDLS in 5 cases. Immunohistochemical studies were performed using the dextran polymer method (EnVisionTM/HRP; DAKO Japan, Tokyo Japan). For immunohistochemistry, 5-μm thick paraffin sections were placed onto silane-coated glass slides. After deparaffinization in xylene, antigen activation was performed by autoclaving for 20 min (121°C). Nonspecific antibody binding was blocked using a specific blocking reagent (0.03% H2O2, sodium azide) for 10 min at room temperature. Sections were incubated with mouse monoclonal antibody against LRP (Clone 5A6 mouse monoclonal immunoglobulin; PROGEN, Toowong, Australia) and against PPAR-γ (Clone 6E3-F1 mouse monoclonal immunoglobulin; Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. At incubation, primary antibodies were diluted in 1:200 (LRP) and 1:50 (PPAR-γ) in Tris-buffered saline (TBS) (DAKO Japan). Negative controls were performed by substituting normal mouse serum for primary antibodies. As controls for positive LRP and PPAR-γ staining, human liver tissue and human breast carcinoma tissue were used, respectively [4, 20, 21]. After washing in TBS, sections were incubated with polymer reagent (Labeled Polymer HRP; DAKO Japan) for 30 min at room temperature. The complex was visualized using 3,3’-diaminobenzidinetetrahydrochloride (DAB) (DAKO Japan). Sections were counterstained with hematoxylin. We selected 5 most-typical sections in each case. If the samples of cases did not have enough volume for making 5 sections, all sections were evaluated. Immunohistochemical staining for antigens was evaluated using a modified method of Nawa et al. [10, 22]. In brief, tumor cells showing a definite staining pattern were scored as positive, regardless of staining intensity. Areas containing the largest number of positive cells were selected and numbers of positive cells per 600 tumor cells were counted. Percentages of positive cells were calculated as the positive rate. If the number of cells failed to reach 600, the number of positive cells as a percentage of the total countable tumor cells was calculated as the positive rate. For dedifferentiated cases, the well-differentiated area and dedifferentiated area were evaluated separately. For statistical analyses, the Kruskal-Wallis test, Steel-Dwass test and Mann-Whitney U test were used. Values of P<0.05 were considered significant. All patients were informed that the surgical specimen would be subject to this study, and provided written form consent.
This study was approved by the institutional ethics committees, and informed consent was obtained from each patient.
RESULTS
Using a monoclonal antibody directed against the LRP, we found LRP in the lipomatous tumor of 50 patients (92.6%) (Fig. 1). No immunostaining was observed after the omission of primary antibody (data not shown). Significant differences in LRP were confirmed among 7 groups. The positive rate was significantly higher in WDLS, PLLS and DDLS than in lipoma. No significant differences were identified between WDLS and the area of WDLS in DDLS (Fig. 2).
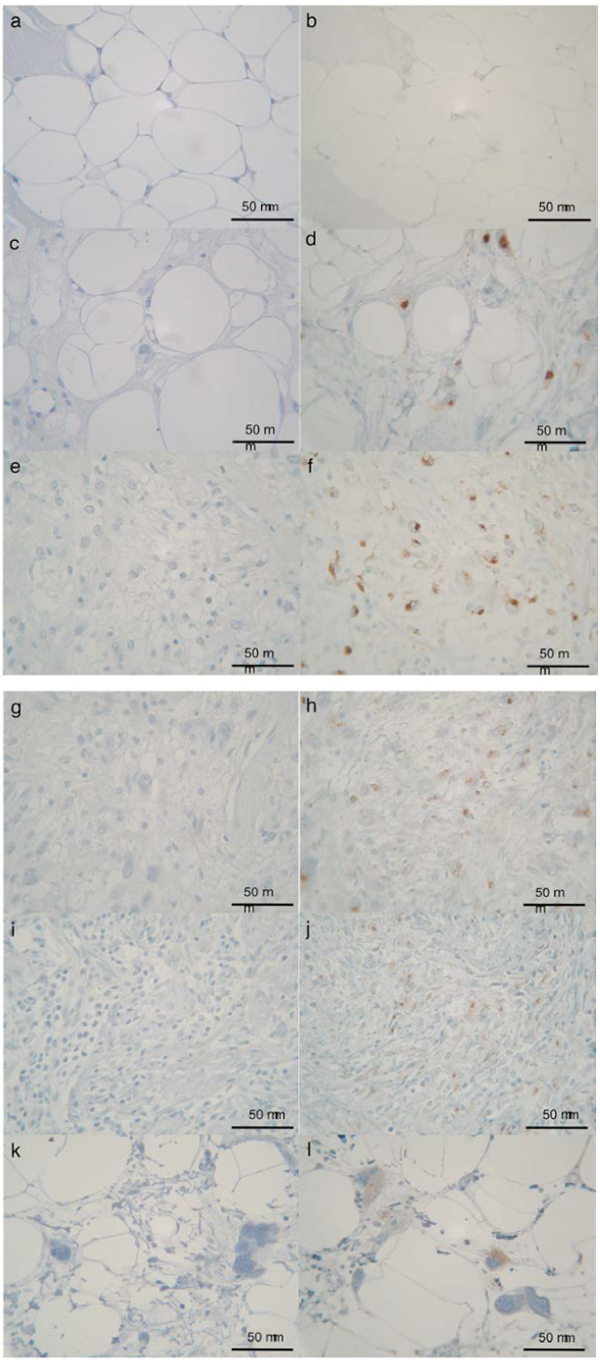
Immunohistochemical expression of LRP in lipoma (b), WDLS (d), M/RLS (f), PLLS (h), DDLS (j) and DDLS-WDLS (l). Negative control (normal mouse serum) of each sections (a, c, e, g, i, k). Original magnification ×400. Positive expression was seen in 50 patients (92.6%).
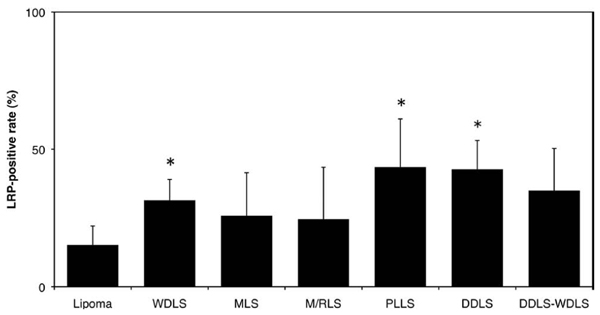
Average LRP-positive rate. Results are shown as means and standard deviation (SD). DDLS-WDLS: Area of WDLS in dedifferentiated liposarcoma. * P<0.05. The positive rate was significantly higher in WDLS, PLLS and DDLS than in lipoma.
We found PPAR-γ in the lipomatous tumor of 44 patients (81.5%) (Fig. 3). No immunostaining was observed after omission of the primary antibody (data not shown).
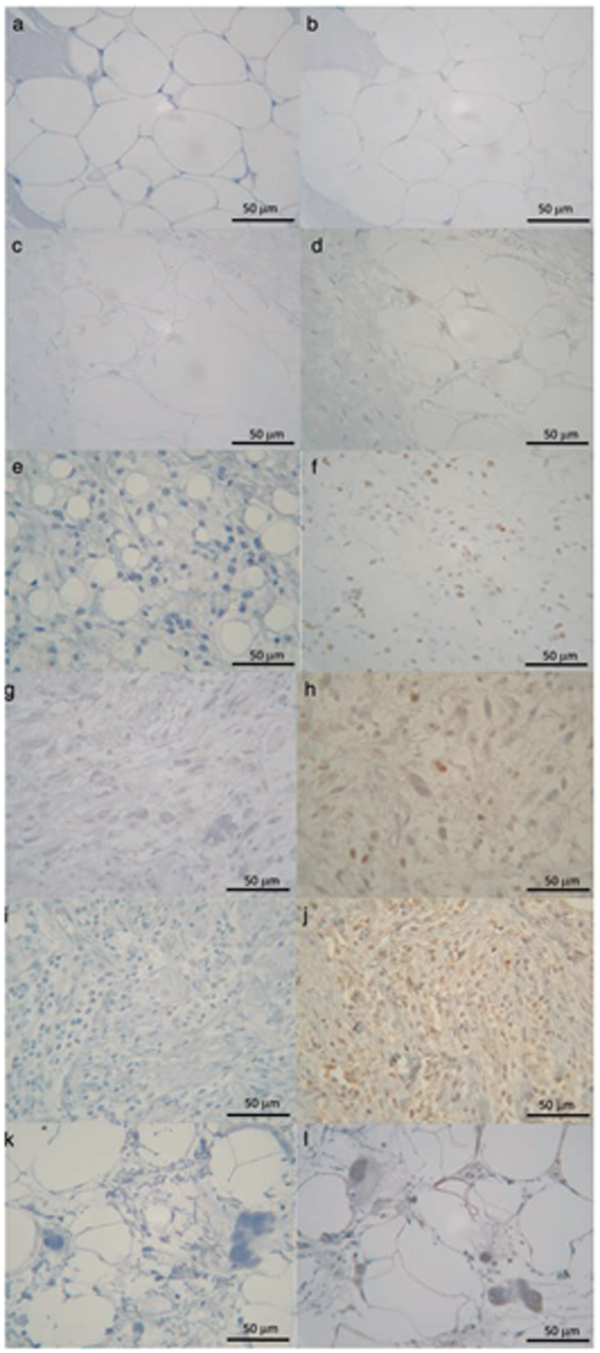
Immunohistochemical expression of PPAR-γ in lipoma (b), WDLS (d), M/RLS (f), PLLS (h), DDLS (j) and DDLS-WDLS (l). Negative control (normal mouse serum) of each sections (a, c, e, g, i, k). Original magnification ×400. The positive expression was seen in 44 patients (81.5%).
Significant differences in PPAR-γ expression were seen among 7 groups (Fig. 4). No significant difference in expression level was apparent between lipoma and WDLS. Compared with the expression rate in lipoma or WDLS, a significant elevation in positive rate was confirmed in MLS, M/RLS, PLLS, DDLS and the area of WDLS in DDLS. Interestingly, a considerably high expression rate was confirmed in both DDLS and the area of WDLS in DDLS.
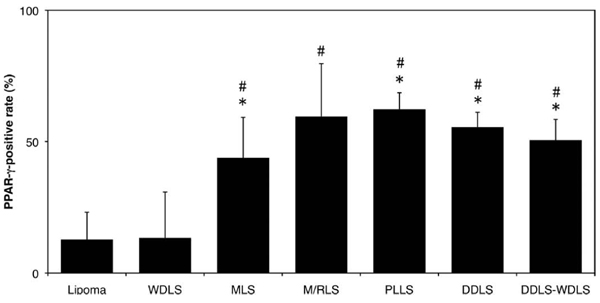
Average PPAR-γ-positive rate. Results are shown as mean and standard deviation (SD). DDLS-WDLS: Area of WDLS in dedifferentiated liposarcoma. * P<0.05: Positive rate was significantly higher in MLS, PLLS, DDLS and WDLS in DDLS than in lipoma. # P<0.05: Positive rate was significantly higher in MLS, M/RLS, PLLS, DDLS and WDLS in DDLS than in WDLS.
Correlations of expression rates between these two molecules were also analyzed, confirming a significant correlation between the two molecules (p = 0.004) (Fig. 5).
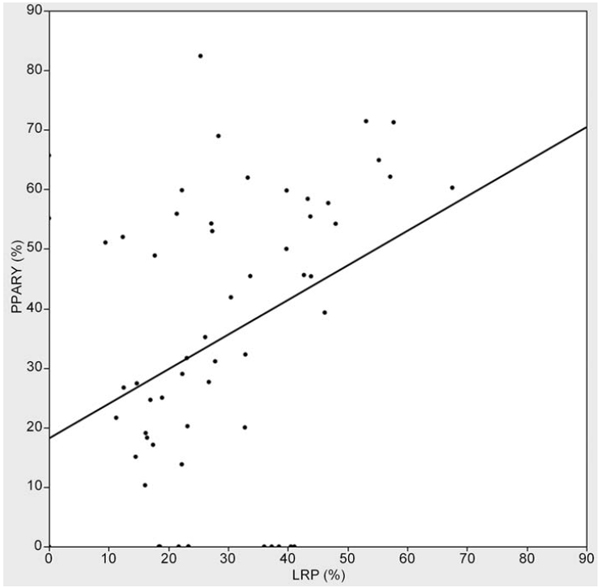
Correlation of expression rate between LRP and PPAR-γ (p=0.004, R2=0.135).
DISCUSSION
The present study has shown the up-regulation of LRP and PPAR-γ, both of which are believed to be critical regulatory molecules of lipogenic differentiation, in high-grade, i.e., less-differentiated lipogenic tumors, than in low-grade tumors.
The regulatory function of LRP on tumor was identified on the basis of the following; distinct expression in various malignant tumor; ligation with MMP or uPA, which are critical for tumor invasion [4-8]. However, the actual role of this molecule in malignancy, i.e., whether inhibitory or progressive, remains controversial due to variations in the relationship between expression level and tumor grade [6, 7, 8, 23]. The origin of such controversial aspects is, at least partly, thought to arise from the multi-functional properties of this molecule [22, 24]. Variety of parameters might change the role of this molecule in regulating tumor activity, resulting in controversial aspects of the properties of the molecule. In the interpretation of the molecular expression in lipogenic tumor, multifactorial functions of the molecule both in terms of fat metabolism and differentiation and of regulatory activity of tumor should have been considered. We first planned this study under the hypothesis that the molecule could regulate fat differentiation in lipogenic tumor, i.e. higher expression in low-grade or benign cases. However, the results revealed an inverse trend. LRP might thus act as a tumor progression regulator rather than in the differentiation of the fat lineage in the system. Previously, up-regulation of uPA expression has been noted in the invasive front or cases of myxoid liposarcoma with worse prognosis (Morii T, et al. unpublished data in English literature), suggesting a close relationship between LRP and tumor progression.
A recent report has suggested a regulatory function of PPAR-γ ligands for LRP expression [15]. Interestingly, a significant relationship between expression rates was identified between these two molecules in the present study, supporting the above-mentioned hypothesis. Further systemic analysis of the proteolysis-internalization system comprising LRP and related proteolytic enzymes, together with PPAR-γ function in regulating LRP in terms of both tumor activity and fat differentiation, should be performed in the future.
Overexpression of PPAR-γ in high-grade cases or malignancy has been reported [25-28]. In vitro analyses showing that antagonism of this molecule results in cell growth inhibition support these results [13, 25]. In contrast, other reports have found PPAR-γ to be expressed more often in cases showing low grade or better prognosis, rather than in those with high grade or poor prognosis [20, 29]. In the present study, expression of PPAR-γ was shown to be elevated according to the malignancy of the lipogenic tumor. A previous report has confirmed the expression and up-regulation of m-RNA of this molecule in lipogeneic tumor [10]. However, that report did not show the relationship of expression intensity and tumor grade or differentiation, perhaps because of a smaller number of cases, unlike the present study. Up-regulation of this molecule in high-grade and less-differentiated cases has evoked several enigmas as to the function of this molecule in lipogenic tumor, because this molecule was, at least originally, believed to have a regulatory function in fat differentiation. As with LRP, we initially hypothesized that down-regulation might be confirmed in less-differentiated cases.
PPAR-γ also has multiple functions, including acting as a promoter of lipogenesis, inflammation regulator and tumorigenesis promoter. PPAR-γ ligands such as troglitazone induce growth arrest not only in macrophages and endothelial cells, but also in several cancer cells such as prostate and breast cancer cells both in vitro and in vivo [30, 31]. Where expression is elevated in low-grade cases, some authors have attributed the phenomenon to activation of the ligand-receptor system that suppresses tumor activity [20]. This hypothesis seems plausible, but most reports of PPAR-γ expression in malignancy have revealed the converse, supporting the results of the present study. Although the precise reasons for this discrepancy are difficult to clarify, some authors have speculated that considering the diversity of human cancer, expression of PPAR-γ may be dependent on tissue specificity and/or the mutational events requisite for cancer development [26, 32].
Although still under debate in some aspects, the role of PPAR-γ in tumor growth inhibition has been extensively studied in terms of experimental, clinical and etiological aspects during the last several years. Many experimental model studies have demonstrated that PPAR-γ ligands are anti-tumorigenic due to anti-proliferative, pro-differentiation and anti-angiogenic effects [13, 25, 33-35]. Moreover, etiological analysis on a database from ten Veteran Affairs medical centers in the United States revealed a strong association between the use of pioglitazone and reduced risk of lung cancer [36].
The present data suggested the possibility of clinical application of these immunohistochemical methods as strong tool for the evaluation of malignancy in lipogenic tumors. Tumors with high expression of PPAR-γ can thus be candidates for molecular targeted therapy through the application of PPAR-γ ligands that can suppress tumorigenesis or tumor proliferation. In fact, a recent clinical trial showed the probability of this receptor as a target of tumor suppression in liposarcoma [10, 37], suggesting tumor suppression in patients with intermediate/high-grade liposarcoma through the induction of differentiation by the PPAR-γ ligand. The subject in the present study was consistent with the largest range of lipomatous tumors and showed a broad range of considerably higher expression for this receptor in high-grade cases than in low-grade or benign cases, supporting the potential of this approach as a promising and attractive strategy.
ACKNOWLEDGEMENTS
We appreciate the helpful advice and expertise provided by Prof. Kazuhiko Satomi and Prof. Yasunori Fujioka. We wish to thank Ms. Mizuho Kosuge and Ms. Miyuki Murayama for technical assistance, and Ms. Ayumi Sumiishi of the pathology department for antibody and tissue collection.


