All published articles of this journal are available on ScienceDirect.
One-Day vs Two-Day Epidural Analgesia for Total Knee Arthroplasty (TKA): A Retrospective Cohort Study
Abstract
Introduction:
Over 500,000 total knee arthroplasties (TKAs) are performed annually in the US, yet postoperative pain management varies widely. In patients managed with epidural analgesia, the epidural catheter is generally removed on the second postoperative day. We compared in-hospital outcomes associated with removing the epidural catheter on postoperative day 1 (POD1-group) vs on postoperative day 2 (POD2-group) among patients undergoing TKA.
Methods:
We identified 89 patients who had TKA performed by a single surgeon from January through July 2007, and who were managed with epidural analgesia. This study took advantage of a change of policy from removing the epidural on the second postoperative day prior to March 2007 (n = 34) to removing the epidural on the first postoperative day thereafter (n = 55). Data were obtained by medical record review and analyzed with bivariate and multivariate techniques. Outcomes included knee range of motion (ROM), pain (0-10 scale), distance walked, narcotic usage, and length of stay.
Results:
The mean patient age was 68 ± 10 years. We did not identify clinically important differences in preoperative characteristics across groups. Patients in the POD1- group had a shorter length of stay (median of 3 vs 4 days in the POD2-group, p<0.001). The POD1-group also walked a greater distance on the second postoperative day (mean of 38 feet vs 9 feet in the POD2-group, p < 0.002). We did not observe a difference between the two groups with respect to change in passive ROM, pain on the second postoperative day, or narcotic usage. The POD1-group had more restricted continuous passive motion settings on the second postoperative day than the POD2-group (50° vs 65°, p = 0.031), and the POD1-group had somewhat worse passive range of motion at discharge (e.g. passive flexion 82° vs 76° in the POD2- group, p = 0.078).
Conclusion:
The balance between a shorter hospital stay and earlier walking achievement with the POD1 -strategy-- vs better ROM at the time of discharge with the POD2-strategy-- should be considered when planning TKA pain management. These results should be confirmed with longer term studies and randomized designs.
Evidence Level III:
Retrospective comparative study.
INTRODUCTION
More than 500,000 total knee arthroplasty (TKA) procedures are performed annually in the United States [1]. Between 1990 and 2002, the number of TKAs almost tripled [2]. By 2030, the demand for TKA is estimated to increase to 3.5 million procedures annually [1]. Recovering from TKA is painful for patients and postoperative pain impedes early physical therapy [3]. Hence, postoperative pain management is a key priority. Despite the large volume of TKAs performed, postoperative pain management strategies vary substantially.
Patients undergoing TKA receive either general or regional anesthesia. Postoperative pain management options include intravenous patient-controlled analgesia (PCA), epidural infusion, and parenteral and oral opioids. One meta-analysis compared neuraxial (spinal or epidural) blockades to general anesthesia across various surgical specialties and noted an overall mortality reduction by a third among patients receiving neuraxial anesthesia [4]. In addition, patients receiving neuraxial anesthesia have been reported to experience fewer serious postoperative complications than patients receiving general anesthesia, including cardiac morbidity (30% fewer complications), pulmonary infections (40% fewer), pulmonary embolism (50%), acute renal failure (30%), and blood loss (30%) [4, 5]. Neuraxial and peripherally-delivered analgesia following TKA reduce the endocrine stress response, provide superior postoperative pain relief and improve joint mobility when compared to parenteral opioids [3, 6-8]. A key advantage of epidural anesthesia is that the catheter can be used to administer both intraoperative anesthesia and postoperative analgesia. Epidural anesthesia and analgesia have been shown to promote early rehabilitation, reduce the length of hospital stay, and decrease the risk of deep vein thrombosis after total joint arthroplasty [9-11]. As a result, epidural anesthesia and analgesia are frequently used pain management strategies for TKA.
The optimal duration of postoperative epidural analgesia has not been clarified. Traditionally, the epidural catheter is removed after 48 hours; longer duration increases the risk of spinal hematoma associated with perioperative anticoagulation [12-15]. However, stopping the epidural infusion on postoperative day two may prolong inactivity resulting in delayed rehabilitation. We hypothesized that by removing the epidural catheter on postoperative day one, rehabilitation milestones, including ambulation, would be attained earlier and length of hospital stay would shorten while pain control would be comparable to the two-day analgesia strategy. To address these hypotheses, we took advantage of a change in clinical policy in one surgeon’s practice to compare the inpatient clinical and healthcare utilization outcomes of two groups of patients who underwent TKA. The first group had epidural catheters removed on postoperative day one (POD1-group) and the second group had epidural catheters removed on postoperative day two (POD2-group).
METHODS
Design
We conducted a retrospective cohort study that took advantage of a change in practice policy in March 2007. Prior to this point, epidurals were removed on the second postoperative day. After March 2007, the policy was changed to remove the epidural on the first postoperative day. Prompted by the desire to control length of stay, and attributing patients’ poor postoperative day two experiences to the prolonged epidural, the change of practice was initiated to shorten epidural use to a single day.
Study Sample
The study sample was composed of patients who underwent TKA performed by a single surgeon at a large orthopedic referral center in a metropolitan area. Ninety-six consecutive patients were identified from the surgeon’s schedule and their electronic medical records were reviewed. Seven patients were excluded because of inadequate documentation of outcomes in the patients’ medical record (3 patients), or because the patients’ epidural analgesia was stopped prematurely (4 patients). Thirty-four patients had the epidural removed on postoperative day two (POD2-group); these patients underwent TKA between January and February of 2007. By the summer months, the change to the epidural practice had been fully operationalized in the clinic. The POD2-group was compared to 55 patients with epidurals removed on postoperative day one (POD1-group); these procedures were performed between June and July of 2007. Institutional review board approval was obtained prior to conducting this study.
Intervention
TKA was performed by a single surgeon using the PFC Sigma Cruciate Retaining cemented prosthesis, using a standard medial parapatellar tendon arthrotomy. The surgery was performed with a tourniquet and drains were not used. The anesthesiology service placed the epidural catheter just prior to surgery. The epidural anesthetic generally consisted of lidocaine and bupivicaine and often hydromorphone, with doses titrated to patient weight and tolerance. While the epidural anesthetic was not standardized, the same anesthesia service cared for both study groups in an identical manner with the exception of the shortened epidural duration. Postoperative pain control also did not change between groups. However, oxycontin was initiated before the epidural was removed in the POD1-group, which wasn’t necessarily true in the POD2-group. Patients received either 325 mg aspirin daily or coumadin with INR 1.8-2.3 for three weeks prostoperatively for DVT prophylaxis, with the treatment decision based on the patient’s BMI, history of thromboembolism, and other risk factors for DVT or PE. The physical therapy protocol involved initial evaluation on the first day after surgery. Patients were seen once daily by the physical therapist or physical therapist assistant for thirty to sixty-minute sessions. The therapist adhered to a protocol that emphasized regaining strength, knee flexion and extension range of motion, improving proprioception, increasing independence with transfers, and use of supportive walking devices for level ambulation and stairs. All patients were managed with a continuous passive motion machine for approximately six hours per day in two hour sessions starting the morning after surgery.
Data Sources
We obtained preoperative and perioperative (within inpatient stay) data from surgical, nursing, and physical therapy notes in the medical records. Supplementary narcotic medication usage was collected from pharmacy records over the course of the patients’ hospital stay.
Baseline Characteristics
Baseline characteristics included age, body mass index (BMI), gender, race (Caucasian or non-Caucasian), side of the operation, preoperative ambulation (independent or with walking aid), Charlson comorbidity index score (a validated, weighted sum of the number and severity of medical problems, scored in this analysis as 0-1 or ≥ 2) [16], preoperative living status (alone or not alone), preoperative primary diagnosis (osteoarthritis or other), smoking status, and insurance status.
Outcomes
The primary outcome was length of stay (LOS), measured as the number of days from surgery to discharge. We also considered several patient-based secondary outcomes including pain, functional measures, discharge destination and supplemental narcotic usage.
Pain was assessed by physical therapists during daily activity and was measured on a 0-10 scale, 10 being the worst. For most patients, pain was measured once per day over the course of the hospital stay. In cases where pain was measured more than once per day, we collected the highest (worst) level of pain recorded for that particular day. For this analysis, we compared the two groups’ pain levels on postoperative day two.
Functional outcomes included distance walked on postoperative day two, measured in feet. We also analyzed degree of assistance needed for walking at time of discharge using the response format of the validated Functional Independence Measure (FIM) [17, 18]. This outcome was dichotomized as none or minimum vs moderate, maximum, or can’t perform. Passive range of motion (ROM) was measured using a goniometer. Passive knee flexion and extension data were collected on postoperative day one and at discharge. We also calculated the change in ROM from postoperative day one to discharge for each patient. Continuous passive motion (CPM) settings were compared at postoperative day two.
Supplementary narcotic medication usage was measured by converting the various opiate medication dosages into milligrams of oxycodone-equivalents. We used oxycodone-equivalents because this drug was the most frequently used analgesic in this cohort. A table of narcotic conversion data (see Appendix) was developed and verified from a number of sources, including the American Pain Society and Thomson’s Healthcare DrugDex Consult database [19-22]. We compared the average cumulative usage of oxycodone-equivalents on the day the epidural catheter was removed. We also compared use of oxycodone-equivalents between the groups on the day after the epidural was pulled (postoperative day two in the POD1-group and postoperative day three in the POD2-group). Patient-controlled analgesia (PCA) narcotics were not included in the calculation of cumulative narcotics dose, because the retrospective nature of the study precluded us from determining how much of the medication the patient had received. However, we did create an indicator variable distinguishing whether the patient received PCA and we controlled for this variable in our analysis. Narcotics dispensed through the epidural catheter were also excluded from this supplementary analysis because it was not possible to compare the quantity of analgesia between epidural administration and oral/parenteral narcotics.
Analysis
Length of stay was compared between the two groups using the Wilcoxon rank-sum test. Pain scores, walking distance, and CPM settings at postoperative day two, as well as passive ROM at postoperative day one, passive ROM at discharge, and change in passive ROM between postoperative day one and discharge were compared between the groups using the independent samples t-test. Discharge destination and assistance level needed for walking at discharge were compared using the Chi-square test.
Since supplementary narcotic medication was ascertained at multiple time points, repeated measures ANOVA was used to examine the impact of epidural strategy controlling for time. For this analysis, time was the number of days since the catheter was removed. For example, Day 1 represents the first 24 hours since the epidural catheter was removed. Day 2 represents the second 24 hours since the epidural catheter was removed. All of the above analyses were performed using SAS software, version 9.1.
Due to the modest sample size and the fact that LOS was not normally distributed, we analyzed LOS using techniques that do not require that the variable have a normal distribution. Specifically, we chose a robust regression analysis with fixed bootstrap re-sampling to determine the effect of epidural strategy on length of stay. This approach provides accurate estimates and valid 95% confidence intervals even if the assumptions of ordinary linear regression are not met, while maintaining the same interpretability as linear regression. The bootstrap procedure is essentially a series of regressions performed on subsets of the same population. In this case, it was performed using 10,000 replicates. The parameter estimates and standard errors from these multiple bootstrap regression results were used to calculate adjusted means and 95% confidence intervals for the parameter of interest. We built a model that included an interaction term of epidural strategy and discharge destination because we hypothesized that the epidural strategy would have different effects on LOS in subjects discharged home than in subjects discharged to another inpatient facility. We also controlled for insurance status in this analysis, as this variable may affect discharge destination. This analysis was performed using R statistical software, version 2.6.2. [23]
Sample Size
The primary outcome variable was LOS. Prior data from our center suggested the standard deviation in LOS was approximately 0.25 days. Thus, we sought to detect a difference in LOS between the 1-day and 2-day groups of at least 0.25 days. With Type I error of 0.05 and power of 90% this required 22 patients in each group. We included at least 30 in each group to account for errors in these assumptions and to increase power for secondary outcomes.
Source of Funding
The funding sources (Departmental Funds, NIH) played no role in the design or conduct of the study.
RESULTS
Cohort Characteristics
The mean age of the entire cohort was 68 ± 10 years and the mean BMI was 31 ± 7 kg/m2. Seventy-eight percent were female, 84% were Caucasian, 29% percent had a Charlson comorbidity score ≥ 2, and 96% underwent TKA for osteoarthritis. Other baseline characteristics of the sample were similar between the two groups (Table 1) except for smoking status. In the POD1-group, 10% reported being a current smoker and 27% reported being a past smoker, while no study subjects reported being a current smoker and 55% reported being a past smoker in the POD2-group (p = 0.02).
Comparison of Baseline Characteristics Between Epidural Strategies
| Continuous Variables | POD1 (N=55) Mean (SD) | POD2 (N=34) Mean (SD) | All Subjects (N=89) Mean (SD) |
|---|---|---|---|
| Age | 68.6 (9.8) | 66.5 (9.2) | 67.8 (9.6) |
| BMI | 30.7 (7.2) | 32.5 (6.7) | 31.4 (7.0) |
| Categorical Variables | POD1 (N=55) N (%) | POD2 (N=34) N (%) | All Subjects (N=89) N (%) |
| Gender Femal eMale |
44 (80.0%) 11 (20.0%) |
25 (73.5%) 9 (26.5%) |
69 (77.5%) 20 (22.5%) |
| Race Caucasian Non-Caucasian |
46 (83.6%) 9 (16.4%) |
29 (85.3%) 5 (14.7%) |
75 (84.3%) 14 (15.7%) |
| Side of the operation Right Left |
34 (61.8%) 21 (38.2%) |
21 (61.8%) 13 (38.2%) |
55 (61.8%) 34 (38.2%) |
| Preoperative Ambulation Independent Ambulate with walking aid |
33 (60.0%) 22 (40.0%) |
24 (70.6%) 10 (29.4%) |
57 (64.0%) 32 (36.0%) |
| Charlson Comorbidity Index 0-1 ≥ 2 |
39 (70.9%) 16 (29.1%) |
24 (70.6%) 10 (29.4%) |
63 (70.8%) 26 (29.2%) |
| Preoperative Living Status Alone Not alone |
14 (25.5%) 41 (74.6%) |
11 (32.4%) 23 (67.6%) |
25 (28.1%) 64 (71.9%) |
| Preoperative Diagnosis Osteoarthritis Other |
52 (94.5%) 3 (5.5%) |
33 (97.1%) 1 (2.9%) |
85 (95.5%) 4 (4.5%) |
| Smoking Status Current Past Never |
5 (9.6%) 14 (26.9%) 33 (63.5%) |
0 (0.0%) 18 (54.5%) 15 (45.5%) |
5 (5.9%) 32 (37.6%) 48 (56.5%) |
Bivariate Analyses
Length of Stay
Results of the Wilcoxon rank-sum test found that the POD2-group had a statistically significantly greater length of stay than the POD1-group. The median length of stay for the POD2-group was 4 days (mean 4.0, range: 3-7 days), while the median length of stay for the POD1-group was 3 days (mean 3.2, range: 2-5 days; p < 0.001).
Patient Outcomes
Results of the independent samples t-test found no differences between the groups with respect to pain scores at postoperative day two (p = 0.445). The POD2-group experienced better passive flexion at postoperative day one than the POD1-group (mean = 73° and 66° respectively; p = 0.045). The POD2-group also experienced better passive flexion at discharge than the POD1-group (mean = 82° and 76° respectively) although the difference was not statistically significant (p = 0.078). At the first postoperative day, the POD2-group experienced better passive extension than the POD1-group (mean = 6° and 10° lacking full extension respectively; p = 0.013). The POD2-group also experienced better passive extension at discharge than the POD1-group (mean = 4° and 7° lacking full extension respectively; p=0.047). There was no difference between the two groups for change in passive flexion (p = 0.665) or passive extension (p = 0.356) from the first postoperative day to discharge. The POD2-group had a higher mean CPM setting at postoperative day two than the POD1-group (mean = 65° and 50° respectively; p=0.031). The POD1-group walked a greater distance at the second postoperative day than the POD2-group (mean = 38 and 9 feet respectively, p < 0.002). Multivariable linear regression models of these outcomes (pain, range of motion, walking distance) did not alter the effect of epidural strategy on these outcomes when controlling for age, gender, and race. Thus, we have chosen to present just the bivariate analyses.
Results of the chi-square test found no difference between the two epidural strategies with respect to assistance level needed for walking at discharge and discharge destination (p = 0.703 and 0.738 respectively; Table 2). Multivariable logistic regression models did not alter the effect of epidural strategy on these outcomes when controlling for age, gender, and race. Thus, for these analyses as well, we have chosen to present only the bivariate analyses.
Comparison of Outcomes between Epidural Strategies
| Continuous Outcome | POD1-Group | POD2-Group | p-Value | ||||
|---|---|---|---|---|---|---|---|
| Median | Mean | 95% CI | Median | Mean | 95% CI | ||
| Length of stay (in days)* | 3 | 3.2 | (3.1, 3.4) | 4 | 4.0 | (3.7, 4.4) | <0.001 |
| Pain scores at post-op day 2 (0-10, 10 worse)† | 7 | 6.4 | (5.6, 7.2) | 7 | 6.8 | (6.0, 7.6) | 0.445 |
| Continuous passive motion (in degrees) at post-op day 2† | 55 | 49.6 | (40.4, 58.8) | 70 | 64.9 | (55.3, 74.6) | 0.031 |
| Distance walked (in feet) on post-op day 2† | 10 | 37.9 | (24.3, 51.5) | 3 | 9.3 | (2.5, 16.1) | < 0.001 |
| Passive flexion (in degrees) at POD1† | 68 | 66.0 | (61.6, 70.4) | 80 | 73.4 | (67.3, 79.5) | 0.045 |
| Passive flexion (in degrees) at discharge† | 80 | 75.9 | (71.3, 80.5) | 88 | 81.9 | (77.0, 86.9) | 0.078 |
| Change in passive flexion (degrees)†, ‡ | 11.5 | 11.1 | (6.1, 16.1) | 6 | 9.2 | (1.3, 17.0) | 0.665 |
| Passive extension (degrees lacking full extension) at POD1† | 10 | 9.5 | (7.3, 11.7) | 5 | 5.5 | (3.7, 7.4) | 0.013 |
| Passive extension (degrees lacking full extension) at discharge† | 5 | 7.1 | (4.7, 9.4) | 3.5 | 3.9 | (2.2, 5.5) | 0.047 |
| Change in passive extension (in degrees)†, ‡ | 2 | 2.7 | (1.0, 4.3) | 0 | 1.5 | (-0.6, 3.5) | 0.356 |
| Categorical Outcome | N | % | 95% CI | N | % | 95% CI | p-value |
| Independent or minimally assisted walking at discharge§ | 42 | 76.4% | (65.1, 87.6) | 24 | 72.7% | (57.5, 87.9) | 0.703 |
| Discharged to rehab§ | 32 | 58.2% | (45.2, 71.2) | 21 | 61.8% | (45.4, 78.1) | 0.738 |
95% CI in parentheses.
* The Wilcoxon rank-sum test was used to make comparisons between groups.
† Independent samples t-test was used to make comparisons between groups.
‡ Positive values represent an improvement in passive flexion or extension.
§ Chi-square test of independence was used to make comparisons between groups.
Multivariable Analyses
Length of Stay
Results of the robust regression with fixed bootstrap resampling found that the effect of epidural removal strategy on LOS depended on the subject’s discharge destination. LOS was not affected by epidural strategy when the subject was discharged to a rehabilitation facility. The mean LOS for these patients was 3.5 (95% CI: 3.3, 3.8) in the POD2-group and 3.2 (95% CI: 3.0, 3.4) in the POD1-group. However, LOS was affected by epidural strategy when the subject was discharged to their home. The mean LOS for these patients was 4.5 (95% CI: 4.2, 4.9) for the POD2-group and 3.3 (95% CI: 3.1, 3.6) for the POD1-group (Fig. 2). We did not find an association between the insurance status of the patient and LOS in this analysis.
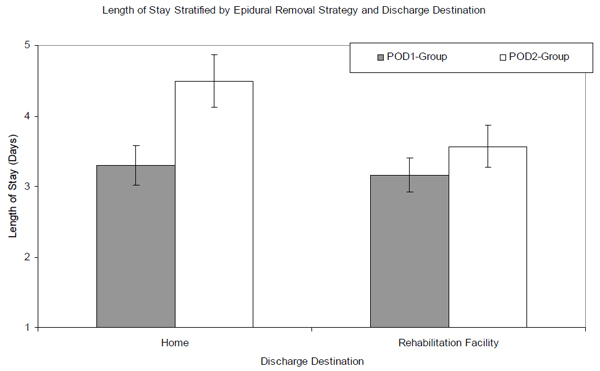
Title: Mean length of stay stratified by epidural removal strategy and discharge destination. Mean length of stay, stratified by epidural-removal strategy using the robust regression with the bootstrap (10,000 replicates), are represented by the bars. The gray bars represent the POD1-group, while the white bars represent the POD2-group. The error bars represent the lower and upper bounds of the 95% confidence interval.
Supplementary Narcotic Medication Usage
We compared the amount of supplementary narcotic usage between the two groups on the day the epidural catheter was removed as well as the amount used on the following day. Thirty-five percent of patients in the POD1-group and 59% of patients in the POD2-group were on PCAs on the first day the epidural catheters were removed (Fig. 1). Results of the repeated measures analysis did not find a significant interaction between time since the epidural catheter was removed and the epidural strategy on narcotic usage (p = 0.249), controlling for whether or not the patient used PCA. On the day the catheter was removed the POD1-group used 108 mg of oxycodone-equivalents, while the POD2-group used 81 mg of oxycodone-equivalents. On the following day, the POD1-group had used 165 mg, while the POD2-group had used 121 mg. These differences between the groups were not statistically significant since the 95% confidence intervals overlap (Fig. 1).
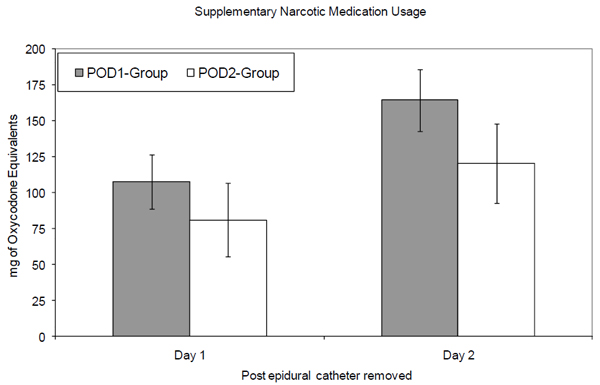
Comparison of supplementary narcotic medication usage following epidural catheter removal by group. Mean cumulative narcotic usage in milligrams of oxycodone-equivalents are represented by the bars with the error bars representing the lower and upper bounds of the 95% confidence interval. The gray bars represent the POD1-group, while the white bars represent the POD2-group. Day 1 represents the first 24 hours since the epidural catheter was removed. Day 2 represents the second 24 hours since the epidural catheter was removed.
DISCUSSION
Total knee arthroplasty is a frequently performed procedure that predictably relieves pain and restores functional status, but involves a painful postoperative course. Many studies have compared the outcomes of epidural pain management to either general anesthesia with parenteral analgesia or to peripheral block methods [3, 4, 6-10, 24]. However, we are unaware of any studies comparing the effect of duration of epidural analgesia on in-hospital rehabilitation and utilization outcomes following TKA. This study took advantage of a change in practice policy from removal of the epidural catheter on the second postoperative day to removal on the first postoperative day. We performed a retrospective cohort study to compare the postoperative outcomes between patients with epidural catheters removed either on POD1 or on POD2.
Baseline characteristics between groups were quite similar, supporting the validity of comparing these two groups. The POD1-group had a median LOS of three days, while the POD2-group had a median LOS of four days. We found that this effect of epidural strategy on LOS was modified by discharge destination. Subjects that were discharged to home had a shorter LOS in the POD1-group than those in the POD2-group. Length of stay was similar between the two epidural strategies for participants discharged to a rehabilitation facility. While there was no significant difference in the percent of patients discharged to a rehabilitation facility between the epidural strategy groups, both POD1- and POD2-groups had higher rates of discharge to rehabilitation facilities than the national average.
This study demonstrated that patients with an epidural removed on the first postoperative day were mobilized sooner and discharged after a shorter hospital stay than patients whose epidural catheters were removed on the second postoperative day. The POD1-group also walked a greater distance than the POD2-group on the second postoperative day. We did not observe a difference in reported pain or need for assistance for walking at discharge. Patients in the POD1-group had worse range of motion on the first postoperative day. Typically, the epidural catheter was removed in the early morning and the physical therapist measured ROM in the afternoon. By the time ROM was measured the POD1-group had lost several degrees of flexion and extension. This difference was maintained throughout the hospitalization, such that the POD1-group had somewhat worse ROM at discharge. Whether this difference is maintained or ultimately dissipates is an important question for future work.
We note several limitations. Neither physical therapists who assessed mobility, functional status and pain nor study participants were blinded to the epidural removal strategy. They were not, however, aware that this comparison was going to be conducted. Furthermore, the study did not utilize a random allocation of study participants to epidural removal strategies under consideration. The quasi experimental design did result in well-balanced groups, however. While we showed that Medicare insurance status did not affect LOS, a larger study could have examined the association between payer and LOS in greater detail. Data were collected as part of routine clinical care and not under a research protocol. One consequence is that outcome data were not available for three patients, introducing possible bias. It is conceivable that different times of year in which the two groups were admitted (POD2-group in winter, POD1-group in summer) were associated with distinctive outcomes. Data on problems in other joints were not available but would also have been helpful to include in the multivariate analysis. Incomplete data in the medical records, particularly the rehabilitation and pharmacy notes, and lack of preoperative range of motion also limited the information available for this study.
CONCLUSION
Longer term follow-up, randomized design and a cost-effectiveness analysis are needed to more rigorously evaluate the outcomes of these two analgesic strategies, and the relationship between program costs and quality of life benefits. The balance between a shorter hospital stay and earlier walking achievement with the POD1-strategy -- vs better ROM with the POD2-strategy-- should be considered when planning TKA pain management.
ACKNOWLEDGEMENTS
Supported by the Department of Orthopaedic Surgery, Brigham and Women’s Hospital; National Institutes of Health (NIAMS) K24 AR 02123, P60 AR 47782.
APPENDIX
Analgesic Equivalents and Conversion Factor to Oxycodone-Equivalents


