All published articles of this journal are available on ScienceDirect.
Significance of Elevated Blood Metal Ion Levels in Patients with Metal-on-Metal Prostheses: An Evaluation of Oxidative Stress Markers
Abstract
It is widely known that cobalt and chromium ions can enhance the production of reactive oxygen species, known to be damaging to cells by disturbing their redox status and then generating oxidative stress. The aim of the present study was to determine if increased metal ion levels induce a state of oxidative stress in patients with metal-on-metal (MM) hip arthroplasty. Results indicated that there was no significant difference in the concentration of oxidative stress markers (total antioxidants, peroxides, and nitrated proteins) in the patients with MM bearings compared to patients without prostheses. The activity antioxidant enzymes was stable (catalase and glutathione peroxidase) or slightly decreased (superoxide dismutase and heme oxygenase-1) over time. This work is the first to determine the biological effects of metal ions released from MM hip implants with regards to mid-term systemic oxidative stress and showed that the increased levels of Co and Cr ions are not associated with significant oxidative stress damage in the plasma of patients with these implants.
INTRODUCTION
Metal-on-metal (MM) hip prostheses, made of cobalt-chromium (Co-Cr) alloys, represent an excellent alternative to metal-polyethylene bearings in the treatment of osteoarthritis of the hip because of their substantially lower wear rates [1]. However, the main concern associated with such bearings is the presence of circulating ions in the organism [2]. These metal alloys undergo corrosion, either electrochemically or mechanically, and release metallic particles and ions that disperse systemically [3]. Indeed, several studies have shown that Co and Cr particles and ions can enter the bloodstream and accumulate both in surrounding tissues and organs of patients after MM total hip arthroplasty (THA) [4-6]. These ions are potentially toxic [7, 8], may cause metal hypersensitivity [9, 10], and chromosomal aberrations [11], and may induce changes in the proportions of peripheral blood lymphocytes [12-14].
The mechanism of metal ion toxicity is not completely understood but it is known that they can generate reactive oxygen species (ROS), such as superoxide ions (O2.-), hydrogen peroxide (H2O2), hydroxyl radical (OH.), and nitrogen oxide (NO.) through Fenton/ Haber-Weiss chemistry [15]. These ROS are known to be involved in numerous human diseases, including degenerative lung and heart conditions, Alzheimer disease, rheumatoid arthritis, and aging [16]. Additionally, ROS are implicated in the induction of oxidative stress generating cell and tissue damage. Oxidative stress may result from an increased production of oxidants and/or a decrease in antioxidant defense. Since the damage that a ROS produces depends on its origin and type, the most accurate and clinically relevant measurement of oxidative damage is to measure multiple products of this damage [17].
In the present study, we hypothesized that metal ions released from MM bearings, namely Co and Cr, could generate oxidative stress in the plasma of patients with MM THA. We first measured the concentration of both Co and Cr ions in blood of patients with 28 mm-head MM prostheses. We then evaluated the concentrations of total antioxidants (TAS), peroxides, and nitrated protein as markers of oxidative stress in the plasma of these patients. TAS measures the overall antioxidant capacity of serum samples while peroxides were used as a marker of lipid peroxidation and nitrated proteins (nitrotyrosines) as a marker of damage to proteins. Since the induction of oxidative stress is also related to the activity of specific antioxidant enzymes, we measured in a second step the activity of some of these enzymes in the plasma of these patients. We specifically looked at classical antioxidant enzymes [18] such as catalase (CAT), glutathione peroxidase (GPx), and superoxide dismutase (SOD), as well as the non-classical antioxidant enzyme heme oxygenase-1 (HO-1) [19]. These enzymes were chosen because their expression (CAT, GPX, and SOD) is modulated by Co and Cr ions in human MG-63 pre-osteoblasts in vitro [20] and are implicated in multiple human disease processes [18] and also because the induction of HO-1 is a general response to oxidative stress in mammalian cells [21].
In a recent study [22], it has been showed that the levels of oxidative stress markers are not elevated in the plasma of patients 1 year after the implantation of MM bearings. To the best of our knowledge, the present paper is the very first study of its kind attempting to further understand the clinical significance of elevated metal ion levels by evaluating their mid-term effect at the circulating oxidant/antioxidant level.
MATERIALS AND METHODOLOGY
Study Groups
A total of 127 patients having a 28 mm-head MM articulating interface (Metasul, Zimmer, Warsaw, IN, USA) were included in the study. No funds were received for this study. Exclusion criteria were determined specifically attempting to minimize alternate sources of increased metal ions in blood. Indeed, the exclusion criteria were bilateral hip involvement, infection, any current metal hardware, and severe medical disability limiting ambulation. The study was approved by our Institutional Review Board. Prior ethics committee approval and informed written consent were obtained. The patients were separated into 5 groups: 1) the control group (pre-operative: Pre-OP) includes 46 patients; 2) 40 patients were included in the “less than 6 months” group; 3) 23 patients were included in the “1-2 years” group; 4) 28 patients were included in the “3-4 years” group; 5) 36 patients were included in the “more than 4 years” group. Patients before surgery were chosen as control subjects because they allow comparing groups of similar age and health conditions. The most common diagnosis for surgery in all groups was osteoarthritis (71% to 85% of patients) while rheumatoid arthritis was the cause for surgery in 0 to 12 % of the patients (Table 1).
Diagnosis of Patients in the Different Study Groups
| OA | RA | CDH | AVN | |
|---|---|---|---|---|
| Pre-OP (Control) | 83% | 10% | 7% | 0% |
| ≤ 6 months | 71% | 8% | 13% | 8% |
| 1-2 years | 72% | 6% | 11% | 11% |
| 3-4 years | 85% | 0% | 7.5% | 7.5% |
| > 4 years | 79% | 12% | 6% | 3% |
OA: Osteoarthritis; RA: Rheumatoid arthritis; CDH: Congenital dislocation of the hip; AVN: avascular necrosis.
The age of patients at the time of blood sampling was similar for the 5 groups. The percentage of smokers varied from 14% of the patients (less than 6 months group) to 7% in the control Pre-OP group (Table 2). The Harris Hip Scores (HHS) and the UCLA activity scores were also similar, except for the control Pre-OP and the less than 6 months groups that had lower results.
Demographic Data and Outcome Measures of Patients in the Different Study Groups
| Age (Range) | Male/Female | Smoker | HHS | UCLA | |
|---|---|---|---|---|---|
| Pre-OP | 55 ± 9 (39-76 y) | 23M/23F | 7% | 47 ± 14 | 4.8 ± 2.3 |
| ≤ 6 months | 60 ± 10 (42-71 y) | 8M/32F | 14% | 75 ± 16 | 4.7 ± 1.9 |
| 1-2 years | 56 ± 11 (31-72 y) | 4M/19F | 11% | 85 ± 14 | 6.0 ± 1.3 |
| years | 51 ± 8 (32-64 y) | 12M/14F | 8% | 87 ± 13 | 6.6 ± 1.6 |
| > 4 years | 52 ± 8 (33-65 y) | 21M/15F | 8% | 90 ± 11 | 7.1 ± 1.6 |
Blood Samples and Ion Concentrations
Whole blood samples were collected in Sarstedt Monovette tubes equipped with needles for trace metal analysis (Sarstedt, Montreal, QC) and kept at -80ºC until analysis. Co and Cr ions levels were measured as previously described by inductively coupled plasma mass spectrometry (ICP-MS, PerkinElmer SCIEX Elan 6100 DRC ICP-MS system; PerkinElmer Instruments, Norwalk, CT) at the Geochemical Laboratories of McGill University [22]. The biological reference standard SeroNorm Trace Elements Whole Blood, Level 2 (Sero AS, Billingstad, Norway) was analyzed as a quality-control sample.
Plasma Preparation
Whole blood of patients (1.5 ml) was also collected into Sarstedt Li-Heparin tubes and centrifuged at 500 x g for 10 min. Supernatant (plasma) was stored at -80ºC for later analysis. Plasma was chosen for this part of the study instead of whole blood because the assays for oxidative stress are not recommended for whole blood and can lead to erroneous data.
Oxidative Stress Markers
Total antioxidant status (TAS) was measured in the plasma using the Oxford Biomedical total antioxidant power kit (Oxford, MI, USA). This assay is based on the capacity to prevent the ABTS (2-2’-Azino-di-[3-ethylbenzthiazoline sulphonite]) oxidation by metmyoglobin and has an inter-assay coefficient of variation (CV) of 3%.
Lipid peroxidation was measured as the total peroxide concentration in the plasma using the Biomedica OxyStat assay (Medicorp, Montreal, QC), which assesses the concentration of an orange complex formed by the oxidation of Fe2+ by peroxides contained in samples. The assay has a 5.1% CV inter-assay precision.
Plasma nitrotyrosine levels (nitrated proteins) were quantified using the BIOXYTECH® Nitrotyrosine-EIA assay (OxisResearch™, Portland, OR, USA) that has an 11.2%CV inter-assay precision.
Antioxidant Enzymes
The three types of superoxide dismutases (Cu/Zn-, Mn-, and Fe-SOD) present in the plasma were assessed by a colorimetric assay (Superoxide Dismutase Assay kit, Cayman, Ann Arbor, MI, USA). Cayman’s superoxide dismutase assay kit utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide. It has a 3.2%CV inter-assay precision.
Catalase (CAT) activity was measured in the plasma of patients using the Catalase Assay kit of Cayman, a colorimetric test based on the capacity of CAT to produce formaldehydes after hydrolyzing H2O2. The inter-assay CV is 3.8%.
The Glutathione Peroxidase Assay kit of Cayman was used to determine the activity of all the glutathione peroxidases (GPX) present in patient plasma. This assay measures GPX activity indirectly by a coupled reaction with glutathione reductase and has a 5.7%CV inter-assay precision.
The presence of heme oxygenase-1 (HO-1) was assessed using the Human HO-1 ELISA kit from Assay Designs (Ann Arbor, MI, USA) in which a mouse monoclonal antibody specific to human HO-1 is immobilized on wells and captured HO-1 present in samples. The sensitivity of the assay is 0.78 ng/mL.
Statistical Analysis
TAS, peroxides, CAT, and GPX data were symmetrically distributed. Therefore, ANOVA followed by Scheffe’s test was used to compare the different study groups. Metal ion, nitrotyrosine, SOD, and HO-1 data distributions were asymmetric and variability between groups was significant. The Kruskall-Wallis test that is a nonparametric equivalent of a one-way analysis of variance was used. It is calculated as a regular ANOVA, but uses the ranks of the data and is therefore resistant to outliers represented by the dots (•) in the box-plot visualization of results, in which the box by itself represent the middle 50% (25% to 75% percentiles) of the data. To facilitate calculations, all null values were defined as the half of lowest sensitivity range of the assay. A p value < 0.05 was considered as significant.
RESULTS
Fig. (1) corresponds to the levels of Co and Cr ions in blood of patients with 28 mm-head MM prostheses. Results showed a significant increase of both Co (Fig. 1A) and Cr (Fig. 1B) in the blood of these patients within 6 month after their hip replacement surgery. The level of Co increased from 1.88 ppb in Pre-OP patients to 2.51 ppb in the less than 6 months patients, while the level of Cr passed from 0.35 ppb in Pre-OP patients to 0.72 ppb in the less than 6 months patients. The levels of both Co and Cr ions then reached a plateau at 1 year and stayed stable thereafter.
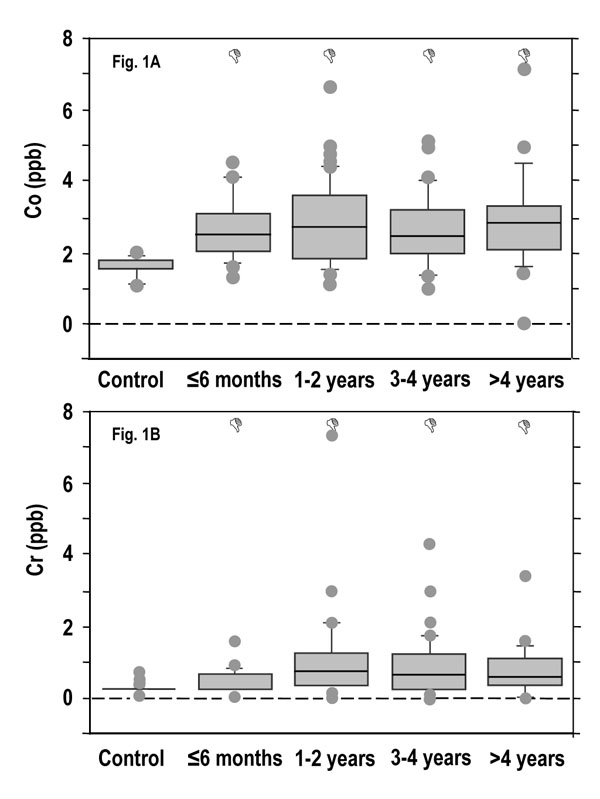
Co and Cr ion concentrations in the blood of patients with MM THAs. Due to non-parametric distribution, results are presented as box plot in which outliers are represented by the dots (•) and the box by itself represents the middle 50% of the data. Δp < 0.05 vs control.
Figs. (2-4) show the concentration of oxidative stress markers in the plasma of patients with 28 mm-head MM prostheses. Results showed that there were no differences between the various time groups for TAS with concentrations varying from 982 to 1398 mM (Fig. 2), peroxides with concentrations varying from 299 to 502 nmol/ml (Fig. 3), and nitrotyrosines with concentrations varying from 115 to 375 nM (Fig. 4), suggesting that the increased levels of Co and Cr ions did not induce oxidative stress damage in the plasma of these patients.

Total antioxidant status (TAS) in the plasma of patients with MM THAs. Results are expressed as the mean ± standard deviation.

Peroxide concentrations in the plasma of patients with MM THAs. Results are expressed as the mean ± standard deviation.
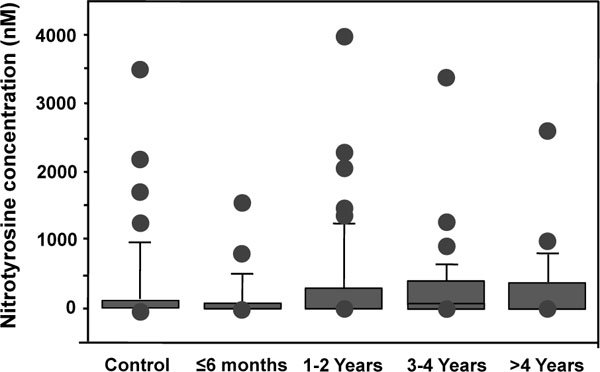
Nitrotyrosine concentrations in the plasma of patients with MM THAs. Due to non-parametric distribution, results are presented as box plot in which outliers are represented by the dots (•) and the box by itself represents the middle 50% of the data.
Figs. (5-8) show the activity of antioxidant enzymes in the plasma of patients. Results showed that SOD significantly decreased by 65.8% in the plasma of patients 1-2 years after surgery (p = 0.005) (Fig. 5). This decrease was transient with a progressive return to control value after 3 years. The activity of SOD in the more than 4 year group was even higher than in the control (p = 0.038), the less than 6 month group (2.4 times; p = 0.0486), the 1-2 year (7.8 times; p = 0.006), and the 3-4 year (1.3 times; p = 0.02) groups (Fig. 5). These results suggest that Co and Cr ions from MM THA may modify SOD activity in the plasma of patients with 28 mm-head MM prostheses. Results also showed slow, but not significant changes in CAT activity for all time intervals (Fig. 6). There were also no statistical differences in the activity of GPX (Fig. 7) between the five groups. Finally, the level of HO-1 decreased after implantation of the prostheses with statistical difference observed at 1-2 year (p = 0.03), at 3-4 year (p = 0.005), and after 4 years (p = 0.009) comparatively to the control group (Fig. 8). In the more than 4 year group, the decrease reached 30% of the control level. The HO-1 level was also lower in the 1-2 year (p = 0.01) and the 3-4 year (p = 0.006) groups compared to the less than 6 month group. These decreases in HO-1 levels suggest a role for Co and Cr ions in the modulation of circulating HO-1 in patients with 28 mm-head MM prostheses.
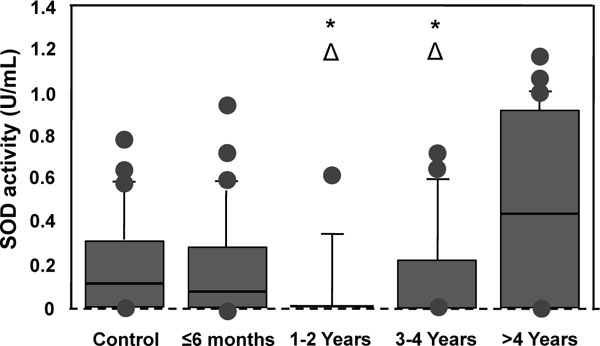
Superoxide dismutases (SODs) activity in the plasma of patients with MM THAs. Due to non-parametric distribution, results are presented as box plot in which outliers are represented by the dots (•) and the box by itself represents the middle of the data. Δp < 0.05 vs control; *p < 0.05 vs ≤ 6 months.
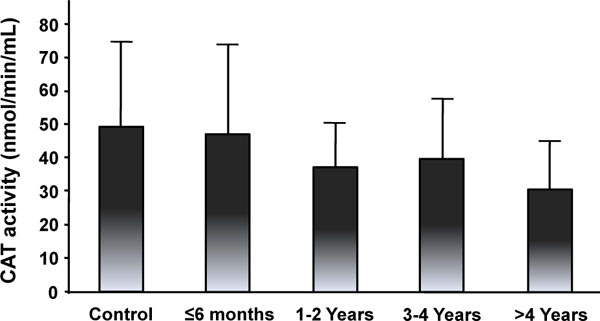
Catalase (CAT) activity in the plasma of patients with MM THAs. Results are expressed as the mean ± standard deviation.
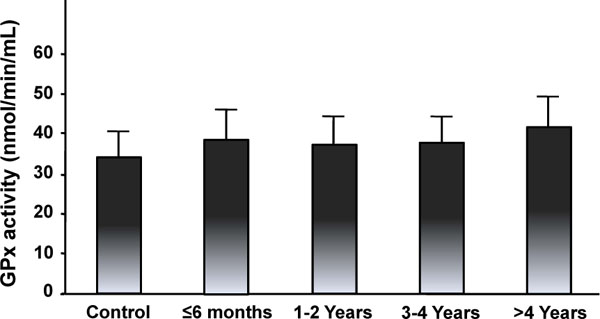
Glutathione peroxidase (GPX) activity in the plasma of patients with MM THAs. Results are expressed as the mean ± standard deviation.

Heme oxygenase-1 (HO-1) concentrations in the plasma of patients with MM THAs. Due to non-parametric distribution, results are presented as box plot in which outliers are represented by the dots (•) and the box by itself represents the middle 50% of the data. Δ p < 0.05 vs control; *p < 0.05 vs ≤ 6 months.
DISCUSSION
The present study showed that levels of Co and Cr ions increased rapidly after surgery in the blood of patients with 28 mm-head MM THA. The levels of both Co and Cr ions in our study were in the same range as those reported in previous studies [23-30] using different prostheses, with mean or median Co ion levels varying from 0.7 to 2.2 ppb (12 to 38 nmol/L) and mean or median Cr ion levels varying from 1.3 to 2.8 ppb (24 to 53 nmol/L). However, due to differences in the choice of samples (erythrocyte vs serum vs whole blood) and in the methodology (digested samples vs diluted samples; ICP-MS vs graphite furnace), it is impossible to identify a prosthesis that releases the least amount of ions into patient circulation.
The rapid increase in blood ions found in the first year is also comparable to what was observed by other groups [28-30]. This increase was followed by a plateau by 4 years. This suggests that, in vivo, the run-in phase of accelerated wear is indeed limited to the first 2 years postoperatively. A steady-state phase was reached from 6 months after surgery. In the present study, the number of patients per group and the fact that the study is not longitudinal (as the groups are formed of different patients) did not allow confirmation of this plateau. The similarity between previous longitudinal studies [28-30] and the present study support the validity of our results even if their non-longitudinal aspect may represent a bias in the analysis of metal ions. The fact that the male/female ratio is not the same in all the study groups represents another limitation of the study. However, a previous study showed no differences between the levels of metal ions in males and females and patients with surface replacements [22] and this ratio discrepancy probably have any effect on the general trends of the data. Results from the present study have in counterpart the advantage of having been obtained with the same method of measurement (needles, tubes, solutions, standard, and technology (ICP-MS)) up to 10 years post-operatively. Indeed, when we subdivided the more than 4 years group, there was no statistical differences in the levels of Co and Cr ions between the 5-6 years group (22 patients) and the ≥ 7 years group (14 patients) (p > 0.8) (results not shown). This remains to be confirmed with larger groups of patients.
The single most significant obstacle preventing a broader application of MM hip arthroplasties and resurfacings continues to be the concerns regarding elevated metal ion levels in the blood, urine, and tissues of patients with these bearing surfaces, especially in young patients [2]. An elevation of chromosomal aberrations was described in peripheral blood lymphocytes within 2 years postoperatively in patients with MM implants. However, as mentioned by the authors themselves, chromosomal aberrations are not necessarily associated with increased cancer incidence [31]. Moreover, the same group also showed chromosome aberrations in patients with metal-polyethylene implants requiring revision [32]. Visuri et al. initially reported a relative higher incidence of hematopoietic cancers in patients with MM prostheses [33]. In the later study, however, the patient population was relatively small and no adjustments were made for co-morbidities that increase the patient risk of developing neoplasm (such as rheumatoid arthritis, Paget’s disease, or bone infarcts). More recently, in a thorough evaluation of a larger cohort of patients, Visuri et al. were unable to demonstrate a relative increased risk of cancer in patients with MM prostheses [34]. Finally, in a meta-analysis of 8 epidemiologic studies including over 130,000 patients with total hip and knee arthroplasties, no causal link could be established between MM hip bearings and cancer [35]. Nevertheless, continued surveillance of patients with MM bearings is warranted to understand the clinical relevance of raised metal ions in these patients.
In an ongoing need to assess the potential consequences of elevated ion levels, we chose to evaluate oxidative stress markers in the plasma of our patient population with MM THAs. Metal ions have the potential to induce the production of reactive oxygen species (ROS), making them prime suspects for disturbing the balance of oxidants/antioxidants in circulating cells [15, 36, 37]. This oxidant/antioxidant unbalance has been identified in numerous degenerative processes including Alzheimer’s disease, macular degeneration, rheumatoid arthritis, neoplasm, and aging [16]. In the present study, we showed that there were no changes in the levels of three oxidative stress markers (TAS, nitrotyrosine, and peroxides) and few changes in the activity of four antioxidant enzymes in the plasma of patients with 28 mm-head MM prostheses compared to the control group, suggesting that the increased levels of metal ions in patients with MM THA had no or little effect on oxidative stress in the plasma of these patients. The results also stayed the same if we subdivided the more than 4 years group into a 5-6 years group and a more than 7 years group as observed for metal ions. Our results suggest that the increased levels of metal ions were not sufficient to disturb the overall defense antioxidant system (as measured by TAS) of patients with 28 mm-head MM prostheses. Notwithstanding the nonexistence of significant mid-term systemic oxidative stress, oxidative stress and/or pathologies in peri-prosthetic tissues cannot be excluded.
Oxidative stress occurs when the balance of formation of oxidants exceeds the ability of antioxidant systems to remove ROS. Actually, it was shown that Co and Cr could modify the expression of CAT and GPX in human MG-63 pre-osteoblasts [20] and that the lack of SOD in knockout mice has dramatic repercussions on phenotypes with perinatal or early postnatal mortality [38]. Moreover, these enzymes are involved in many pathophysiological processes including neurodegeneration, cancer, and aging [18, 39]. In this regard, the antioxidant status and the levels of oxidative stress markers are altered in patients with osteoarthritis [40, 41] and rheumatoid arthritis [41, 42] this may affect results of the present study. However, patients from all groups have similar diagnosis and this has probably a very small impact on the results. Results of the present study show that the overall capacity of plasmatic antioxidant enzymes to remove ROS was not affected by metal ion levels in patients with 28 mm-head MM prostheses. We observed a transient decrease of SOD at 1-2 years postoperatively with a return to control values thereafter. The SOD activity we measured in the plasma corresponded to SOD3 (extracellular SOD) that is present in low concentration compared to cellular SODs. Furthermore, SOD3 present in extracellular spaces of tissues accounts for 90-99% of the SOD3 present in the body. This low concentration of SOD3 circulating in plasma may make it more sensitive to minor changes of oxidative status [43]. This may explain the variation of SOD activity observed in plasma of patients with 28 mm-head MM prostheses whereas the other antioxidant enzymes were not affected in the same conditions. Our results are in agreement with a previous study in COS7 cells where cobalt chloride led to a decrease in the resistance to oxidative damage by down-regulating the expression of SOD3 [44].
Interestingly, it was shown that Co2+ decreased the expression of CAT in a time- and dose-dependent manner, while Cr3+ increased its expression in osteoblast-like cells in vitro [20]. In the present study, we observed a tendency to decreased CAT activity in the plasma of patients with MM prostheses. This suggests that metal ions from MM prostheses, especially Co2+, may affect plasma CAT activity at long-term. The activity of CAT in long-term follow-up remains then to be investigated.
HO-1 is also known to be implicated in many human diseases such as Alzheimer and inflammatory diseases [21]. Previous findings showed that Cr(VI) induced the expression of HO-1 in human dermal fibroblasts [45]. This suggests that Cr ions were not implicated in the progressive decrease of HO-1 we observed in patients with MM THA up to 4 years post-operatively. However, HO-1 increased at longer term (> 4 years), suggesting that the effect might be reversible. A down-regulation of HO-1 was shown in vivo in patients with hepatitis C, while other antioxidant enzymes, such as CAT and SOD, were not affected [46]. This suggests that parameters other than the presence of metal ions might be responsible for the decreased levels we observed in patients with MM prostheses.
CONCLUSION
This work is the first to determine the biological effects of metal ions released from MM hip implants with regards to mid-term systemic oxidative stress and showed that the increased levels of Co and Cr ions were not associated with significant oxidative stress damage in the plasma of patients with these implants.


