All published articles of this journal are available on ScienceDirect.
Histopomorphic Evaluation of Radiofrequency Mediated Débridement Chondroplasty
Abstract
The use of radiofrequency devices has become widespread for surgical ablation procedures. When ablation devices have been deployed in treatment settings requiring tissue preservation like débridement chondroplasty, adoption has been limited due to the collateral damage caused by these devices in healthy tissue surrounding the treatment site. Ex vivo radiofrequency mediated débridement chondroplasty was performed on osteochondral specimens demonstrating surface fibrillation obtained from patients undergoing knee total joint replacement. Three radiofrequency systems designed to perform débridement chondroplasty were tested each demonstrating different energy delivery methods: monopolar ablation, bipolar ablation, and non-ablation energy. Treatment outcomes were compared with control specimens as to clinical endpoint and histopomorphic characteristics. Fibrillated cartilage was removed in all specimens; however, the residual tissue remaining at the treatment site displayed significantly different characteristics attributable to radiofrequency energy delivery method. Systems that delivered ablation-based energies caused tissue necrosis and collateral damage at the treatment site including corruption of cartilage Superficial and Transitional Zones; whereas, the non-ablation system created a smooth articular surface with Superficial Zone maintenance and without chondrocyte death or tissue necrosis. The mechanism of radiofrequency energy deposition upon tissues is particularly important in treatment settings requiring tissue preservation. Ablation-based device systems can cause a worsened state of articular cartilage from that of pre-treatment. Non-ablation energy can be successful in modifying/preconditioning tissue during débridement chondroplasty without causing collateral damage. Utilizing a non-ablation radiofrequency system provides the ability to perform successful débridement chondroplasty without causing additional articular cartilage tissue damage and may allow for other cartilage intervention success.
INTRODUCTION
Débridement chondroplasty serves a large clinical need and disease burden afflicting our population [1-5]. The goal is to remove the damaged cartilage that causes a mechanical and inflammatory impairment of joint function and leads to a deterioration of joint health. Smoothing the articular surface can eliminate the mechanical stress-risers that cause symptoms and propagate cartilage damage; removal of the loose surface debris associated with loss of cartilage function decreases the biologic load the joint needs to address. During such treatment, preserving functioning cartilage tissue is important because in situ chondrocytes have shown low expectant tissue regenerative potential at the site of injury and disease. Undesirably, all current débridement chondroplasty techniques induce necrosis and collateral damage to contiguous untargeted cartilage tissue at the treatment site.
Mechanical motorized shavers have been the traditional treatment standard for débridement chondroplasty [6]; however, ablation-based radiofrequency devices are becoming increasingly more popular [7], either alone or in combination with mechanical motorized shavers [8]. When used correctly, ablation-based radiofrequency devices offer more precise tissue débridement, limiting the extent of collateral tissue damage while accomplishing cartilage procedures deemed beneficial to the patient [7-10]. Studies have shown that débridement chondroplasty with ablation-based radiofrequency devices causes less collateral damage and creates a smoother articular surface than mechanical motorized shavers [8, 11-15]. Despite these advantages, the adoption of ablation-based radiofrequency mediated débridement chondroplasty remains limited as many practitioners are uncomfortable causing iatrogenic collateral damage to articular cartilage. Accordingly, some have even retreated from using mechanical motorized shavers for articular cartilage lesions other than large symptomatic chondral flaps.
The advent of ablation-based radiofrequency mediated débridement chondroplasty has brought the issue of iatrogenic collateral damage to a forefront of cartilage research. The mechanism of action of ablation-based radiofrequency treatments is to induce therapeutic tissue necrosis through electrode-to-tissue contact. In many ablation treatment settings, the tissue type or treatment location tolerates imprecise tissue necrosis and collateral damage as accepted sequelae of the surgical procedure. Yet when applied to treatment sites where tissue preservation is important, such as articular cartilage, researchers have echoed the concern of practitioners as to whether such iatrogenic tissue necrosis and resultant collateral damage are counterproductive to treatment goals [16-22]. Concerns such as whether ablation-induced tissue necrosis delays or impairs residual cellular function [9, 10, 17, 23, 24], alters cartilage mechanical characteristics and permeability properties [15, 23], disrupts the function of the proteoglycan-collagen matrix [15, 23], extends cartilage injury [19, 21, 22], or accelerates the natural progression of disease [15, 18, 20] continue to be raised despite the lack of other more effective treatment options to offer patients.
Because of these circumstances, articular cartilage has become a good model to evaluate tissue preservation techniques due to the susceptibility of chondrocytes to perturbation and the tissue's sensitivity to collateral damage from mechanical, thermal, metabolic, and other disturbances. It is clear that before radiofrequency devices can become more widely accepted for tissue preservation treatments, such as débridement chondroplasty, further control mechanisms for the delivery and deposition of radiofrequency energy to tissue are required. Since ablation-based radiofrequency devices leave behind a residual layer of necrotic tissue at the treatment site, their use may not be appropriate in tissue preservation settings exhibiting little repair and regeneration potential such as articular cartilage.
Recently, non-ablative radiofrequency systems have been developed that deliver a low-level of energy to the treatment site through a protected tip architecture inhibiting electrode-to-tissue contact. The goal of these systems is to deliver non-necrosis-based treatments that maximize tissue preservation while achieving desirable clinical outcomes. The design allows, for example, the combination of controlled low-level radiofrequency energy delivery with a gentle non-motorized and non-electrical-based mechanical treatment feature of the protective housing rather than within the active electrode itself as in ablation-based devices. These newer radio-frequency energy systems do not ablate tissue, but instead modify or precondition tissue toward a state amenable for other treatments, such as, in this case, the débridement of damaged articular cartilage.
The purpose of this study is to evaluate the histopomorphic outcome of radiofrequency device use in a clinical treatment setting that requires tissue preservation based upon the method of energy delivery and deposition. Three methods are studied, all of which have been designed for débridement chondroplasty by their manufacturers, including a monopolar ablation-based system, a bipolar ablation-based system, and a non-ablation system. Even though ablation-based radiofrequency systems have been shown to be more precise than traditional mechanical motorized shavers, it remains unclear whether débridement chondroplasty can be an effective tissue preservation procedure through the use of newer non-ablative interventions.
MATERIALS AND METHODOLOGY
Osteochondral specimens were harvested from patients undergoing total knee replacement under an approved Institutional Review Board protocol. The total knee replacement procedures were performed by a single surgeon in the normal course of his practice. The tissue to be normally discarded during the procedure was examined prior to harvest once the knee joint was entered surgically to determine if it met the requirement for study inclusion. Specimens were included that demonstrated an area of uniform surface fibrillation chondromalacia (partial thickness damage) of sufficient size from which four test samples could be obtained from each specimen, each demonstrating geographically similar characteristics. After harvest, specimens were divided into four parts [25] as depicted in Fig. (1). Each part was randomly sequestered into a treatment group and immediately transferred to an ex vivo saline arthroscopic treatment setting. Four treatment groups were established, consisting of one for each radiofrequency system tested and a control.
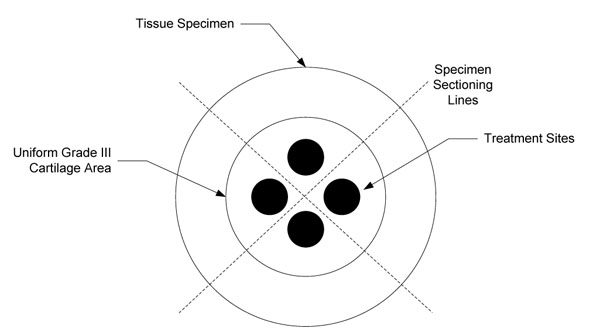
Diagrammatic representation of the specimen division and treatment locations within each segmented part. Specimens were sectioned such that four test samples were obtained, each demonstrating geographically similar characteristics. The treatment sites were away from the sectioned edges but within the uniform surface fibrillation area to avoid edge artifact.
Three radiofrequency systems designed for débridement chondroplasty were studied each utilizing a different method of energy delivery and deposition to tissue. All systems were used per manufacturer's specifications and are characterized in Table 1. They include: Glider® (Smith and Nephew, Inc., Andover, Massachusetts, USA), Paragon® (Arthrocare, Inc.; Austin, Texas, USA), and CeruleauTM (NuOrtho Surgical, Inc., Fall River, Massachusetts, USA). Glider and Paragon deliver ablation-based radiofrequency energy through a monopolar and a bipolar mechanism, respectively, via direct electrode-to-tissue contact. Ceruleau delivers non-ablative radiofrequency energy through a bipolar mechanism initiated from a monopolar generator via a protected tip that prevents electrode-to-tissue contact. Of the three systems, Ceruleau is the only device designed with a mechanical feature for débridement chondroplasty via the protective housing. Glider and Paragon allow mechanical débridement only by the use of the active electrode itself. Each system constituted one of the three treatment groups for each specimen with a fourth group serving as a control that did not receive any treatment.
The Three Radiofrequency Systems Studied
| Device | Glider | Paragon-T2 | Ceruleau |
|---|---|---|---|
| Device Tip Design |  |
 |
 |
| Active Electrode Configuration | Flat electrode; Direct Tissue Contact | Ring Electrode; Direct Tissue Contact | Protected Electrode; Mechanical Housing |
| RF Generator System | Vulcan-EAS Smith and Nephew | Atlas Arthrocare | Force FX-C Valley Lab |
| Electrosurgical Design | Monopolar | Bipolar | Bipolar operating from monopolar output |
| Electrosurgical Mode | CUT | CUT | COAG4 |
| Power Output Settings | 60 Watts | 155-170 Watts | 25 Watts |
| Power Control | Manufacturer Preset Level 27 | Manufacturer Preset Level 6 | Surgeon Controlled |
| Approximate Electrode Surface Area Contacting Tissue 1 | 0.9 mm2 | 2.3 mm2 | Non-Contact |
| Power Density at Tissue Surface 2, 3, | 69 W/mm2 | 67-73 W/mm2 | 2.3 W/mm2 |
1 Approximate surface area measurements of the Glider and Paragon active electrodes available for radiofrequency emittance. Both active electrodes are in direct contact with tissue and deposit energy deep into the tissue's substance. The Ceruleau electrode resides and performs its work within a protective electrically insulating housing that inhibits electrode-totissue contact; therefore, the electrode surface area is not a significant factor at the tissue surface.
2 Power Density equals the power deployed through the exposed electrode surface area and reveals the power density required for direct-contact cartilage tissue ablation. The Glider and Paragon results are remarkably similar indicating a common threshold for inducing cartilage ablation, despite the differences in delivery modality between monopolar and bipolar, which demonstrates that the physical product design can offset energy delivery method.
3 A power density analysis of Ceruleau requires an area measurement of the openings in the protective housing and the distance of the electrode from the tissue surface. The power density at the tissue surface is approximately a 30 fold decrease from that of Glider and Paragon. Ceruleau operates at a higher nominal Voltage in COAG mode; therefore, the current density (the component of power density most responsible for ablation) at the tissue interface is further reduced from that of the ablation-based modalities.
4 Ceruleau is designed for use in a bipolar mode from a monopolar COAG output. These COAG waveforms are biased towards high peak-to-peak voltage levels in comparison to ablation-based systems with a higher current level bias.
Each radiofrequency system was assigned its own station with an ex vivo arthroscopy set-up. Standard saline arthroscopic fluid was used at room temperature with a fluid-flow rate of 30cc/min ± 5cc/min which created consistent fluid dynamics in the set-up typical of in vivo arthroscopy. The flow was measured and recorded at each station throughout the study and was maintained constant for all testing. The Glider and Paragon radiofrequency systems were used at the generator settings recommended by their manufacturer's design. Ceruleau was used at the generator setting determined to be most efficient for débridement chondroplasty even though the system has the ability to fine-tune energy delivery level based upon patient-specific needs.
Débridement chondroplasty was performed by one practicing surgeon accustomed to radiofrequency débridement chondroplasty. The goal of the procedure was to remove the fibrillated and damaged cartilage and smooth the articular surface as determined by visual cues. Motorized shavers or other devices were not used. Energy delivery treatment time was 5 seconds for all specimens with a technique of moving the probe tip back and forth at the treatment site with a consistent application pressure and speed as judged by the surgeon to mimic in vivo treatment conditions [36]. With Glider and Paragon, the surgeon used the active electrode as a mechanical implement for gentle débridement, consistent with their design, by moving the electrode across the articular surface during the allotted energy deposition time. With Ceruleau, the surgeon used the protective housing to gently debride the fibrillated cartilage concurrent with energy delivery for the allotted treatment time.
Immediately after each treatment, three 0.5 mm coronal sections of each sample were obtained referencing the center of the treatment site. The sections were prepared for staining by washing in HEPES buffered saline solution. Live/Dead® Reduced Biohazard Cell Viability Kit #1 “green and red fluorescence”, SKU #L-7013, (Invitrogen™, Carlsbad, California) was used per manufacturer’s specification to stain specimens. Specimens were gluteraldehyde fixed, transferred to standard flat glass slides, and flooded with VectaShield® fluorescence protection oil prior to the placement of #1.5 borosilicate glass cover slips over each specimen section.
Confocal fluorescence laser microscopy analysis was performed by personnel blinded to the identity of the treatment groups for each specimen part. Confocal imaging was performed with an Olympus IX-81 inverted microscope coupled to an Olympus FV300 confocal laser scanning unit (Center Valley, Pennsylvania, USA) using 488 nm laser excitation. Live cells were captured under green fluorescent channel (505-525 nm) and dead cells were captured under red fluorescent channel (577-634 nm), generating a Live image, a Dead image, and an Integrated image.
The effectiveness of the débridement chondroplasty in removing fibrillated cartilage tissue and smoothing the articular surface was assessed by gross visual inspection and microscopic imaging. The extent of collateral damage induced by each treatment was determined based upon both the amount of initially non-damaged cartilage tissue removed from the treatment site when compared to control specimens and the condition of the residual tissue remaining at the treatment site. Because of the difficulty in comparing fibrillation characteristics between patient sample groups, the amount of tissue removed was established by comparing cell density and chondrocyte characteristics of the cartilage's histologic zones relative to control specimens within each group as each zone contains a phenotypically distinct subpopulation of chondrocytes that differs in morphology and distribution within the matrix and chondron. Depth of necrosis of the residual tissue post-treatment was determined by measuring the distance from the center of the residual tissue surface after treatment to the lowest depth of dead cells observed. Multiple range ANOVA analysis was performed for depth of cartilage tissue necrosis and percent chondrocyte death for each device tested.
RESULTS
Six patients yielding six separate specimens originating from femoral condyle resection were included for study, generating twenty-four osteochondral parts tested (n = 6 per Group; total sample parts = 24). All devices performed as suggested by manufacturer without device failure. The final surface morphologies were independently validated based upon visual and tactile cues by two surgeons as satisfactory clinical outcome of removing the fibrillated tissue. However, residual post-treatment tissue characteristics varied significantly between the treatment groups. Figs. (2-5) demonstrate representative images of the specimens for each group.
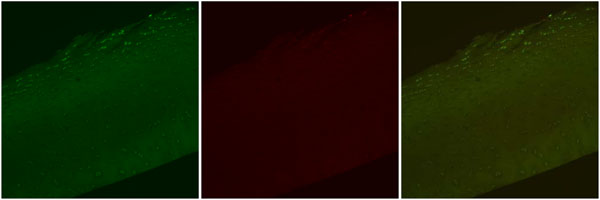
Confocal laser microscopy images, Control. Representative images depicting Live cell stain (green), Dead cell stain (red), and a combined image with both Live cell and Dead cell stain. Note the fibrillation within the Superficial Zone and the normal appearing elongated chondrocytes both within and around the fibrillation. Dead cells are observed only in extruded positions. Original magnification 10x.
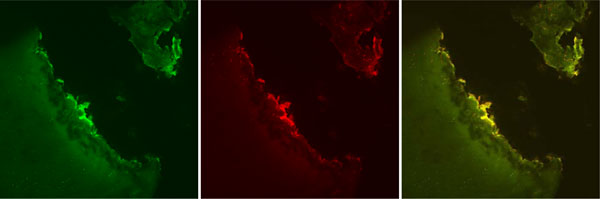
Confocal laser microscopy images, Glider. Representative images depicting Live cell stain (green), Dead cell stain (red), and a combined image with both Live cell and Dead cell stain. Note the level of tissue loss which brings the post-treatment residual necrotic cartilage layer into the Transitional Zone. The Transitional Zone typically demonstrates spherical chondrocytes of less dense population than the Superficial Zone. Original magnification 10x.
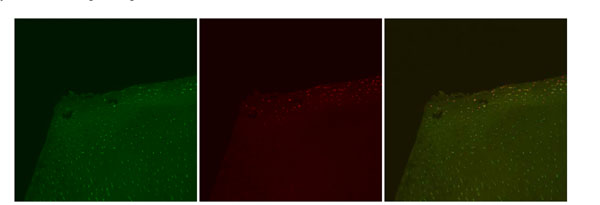
Confocal laser microscopy images, Paragon. Representative images depicting Live cell stain (green), Dead cell stain (red), and a combined image with both Live cell and Dead cell stain. Note that the Superficial Zone remains present, but with significant dead cells observed uniformly throughout this zone and into part of the Transitional Zone. Original magnification 10x.

Confocal laser microscopy images, Ceruleau. Representative images depicting Live cell stain (green), Dead cell stain (red), and a combined image with both Live cell and Dead cell stain. Note the decreased distance of live cells to the surface. These cells exhibit elongated morphology typical of the Superficial Zone. Dead cells are not observed in either the Superficial or Transitional Zone. Original magnification 10x.
The control specimens (Fig. 2) demonstrate typical fibrillated articular surfaces consistent with gross visual inspection of the harvested tissues. The Superficial Zone was clearly disrupted by the fibrillation, but chondrocytes with a flattened chondron appearance typical of this zone remained present even toward the base of the fibrillation. In all control specimens, the fibrillation did not penetrate to the Transitional Zone of the cartilage tissue. Within the fibrillated tissue itself, live cells were observed residing within the tissue at varying distances from the surface of the fibrillation. Live cells were abundantly present in density patterns typical of healthy cartilage around and below the surface fibrillation. Cell population densities within each specimen group remained constant as the samples of each group originated from the same specimen. Inter-specimen comparisons did not reveal significant differences in relative cell population densities, chondron orientation, or cellular distribution patterns confirming similar lesion type included for study. Occasional dead cells were observed to reside in a more extruded position within the fibrillation itself but not within the substance of the morphologically intact cartilage tissue. Adjacent to the fibrillated segments, a more typical softened appearance was observed without fibrillation but with loss of surface cellularity and lacunar emptying within the Superficial Zone below the lamina splendens.
In the Glider treated samples (Fig. 3), large charred tissue segments and loss of cartilage thickness above that of control were observed throughout the treatment site. Tissue charring ranged from light-brownish color to near black or dark grey indicating severe char and tissue damage. There were areas of tissue fragmentation indicating ablation extraction of tissue as is typically observed during standard electrocautery procedures. No treated specimens yielded either a visually or histologically smooth cartilage surface even though the initial fibrillation of the specimens was eliminated. The fibrillated tissue surface was replaced with a residual layer of necrotic and damaged tissue in all instances. No specimen exhibited Superficial Zone characteristics due to the tissue loss that had occurred. Cellular density and chondrocyte characteristics of the residual tissue under the necrotic layer was markedly decreased and consistent with control specimen Transitional Zone cartilage depth, indicating significant loss of tissue exceeding the level of disease pretreatment. Dead cells were present under the charred surfaces in all specimens and occasionally intermixed with live cells. Due to the normally decreased cellular density of the Transitional Zone, the residual tissue under the charred layer exhibited dead cells in a widely distributed pattern within the cartilage matrix indicating significant penetration of the treatment through the extracellular matrix tissue and into deep chondrons. The mean post-treatment depth of necrosis of the remaining residual cartilage at the treatment site was 216.76 µm (range 104.00 µm - 333.79 µm) through the Transitional Zone tissue (as distinct from necrosis through Superficial Zone tissue).
In the Paragon treated samples (Fig. 4), generalized gelatinization of tissue was observed at the treatment site indicative of altered matrix properties. The gelatinized tissue was observed to have a semi-translucent appearance and much softer consistency than the surrounding cartilage. Slightly less bulk loss of normal cartilage was observed when compared to the Glider specimens. In some areas, however, tissue charring in the same geometric pattern as that of the active electrode was visually noticeable in the treatment area. This observation was termed ablation electrode imprinting and was distinctly noticeable due to the unique ring shape of the Paragon electrode. A firmer consistency of the cartilage adjacent to the ablation electrode imprinting was noted with a more typical ablation tissue extraction pattern observed at the site of imprinting. All specimens yielded a surface elimination of the original fibrillated cartilage tissue and a resultant layer of post-treatment necrotic tissue; and, all with some areas of ablation tissue extraction similar to the Glider samples and the goal of tissue ablation. The residual tissue exhibited some latent Superficial Zone characteristics such as elongated chondrons parallel to the articular surface. Abundant dead cells were intermixed with some live cells throughout the entire Superficial Zone in all specimens with additional dead cells observed into the superficial part of the Transitional Zone. The mean post-treatment depth of necrosis of the remaining cartilage at the treatment site was 149.46 µm (range 115.00 µm - 201.29 µm) which straddled the Superficial and Transitional Zones.
In the Ceruleau treated samples (Fig. 5), the fibrillated cartilage tissue was removed resulting in a smooth residual surface at the treatment site. No areas of charred, gelatinized, or color altered tissue were observed at the treatment site consistent with the non-ablative type of energy delivered. All specimens yielded a residual tissue bed demonstrating similar appearance and consistency to that of the non-fibrillated tissue surrounding the treatment site. All specimens retained intact Superficial Zone characteristics in the residual tissue below the level of removed fibrillation without areas of necrosis or dead cells. Accordingly, no specimen part demonstrated additional bulk tissue loss other than that of the diseased fibrillated cartilage. Live cells were evident throughout the residual treatment site with chondrocytes residing closer to the surface than that noted in the non-fibrillated sections of the control specimens of each sample. This finding indicated an increased surface-based level of cellularity post-treatment in the retained Superficial Zone of the treatment site.
Multiple range ANOVA analysis demonstrated a statistically significant difference between the depth of cartilage tissue necrosis (p < 0.005) and percent chondrocyte death (p < 0.004) between the ablation (Glider and Paragon) and non-ablation (Ceruleau) devices, with no statistical difference between the two ablation (Glider and Paragon) devices tested. As depicted in Table 1, the power density levels delivered at the tissue surface by ablation-based devices exceed the energy thresholds that allow chondrocytes to survive.
DISCUSSION
Many studies have supported the efficacy of débridement chondroplasty in relieving patient symptoms by smoothing the articular surface and decreasing the biologic load of joint cartilage debris [26-29]. Within the spectrum of cartilage lesions, the benefit of eliminating mechanical symptoms of cartilage disease is more apparent for traumatic focal lesions [28, 29]; whereas, the benefit of decreasing biologic load is generally more apparent for diffuse osteoarthritic cartilage lesions [26, 27]. Although intuitive that these benefits would contribute to long-term joint health, promotion of cartilage longevity and the mitigation of joint replacement surgery have not yet been clearly shown. Such long-term benefits are difficult to establish due to the large number of variables that need to be controlled, including type of cartilage lesion, concomitant joint pathology such as meniscus and ligament dysfunction, tissue senescence, genetic and inflammatory response variations, downstream mechanical experience, systemic health, and medical treatment history like intra-articular corticosteroids, hyaluronic acid, and oral non-steroidal anti-inflammatory use.
Notwithstanding noting such symptomatic improvement, researchers have questioned the efficacy of inducing damage to normal cartilage tissue during débridement chondroplasty whether by mechanical or ablation-based radiofrequency means [16-22], unless the mechanical symptoms of large chondral flaps are overwhelming. The issue of whether eliminating the symptoms from articular cartilage damage decreases the natural progression and/or disease burden is the subject of ongoing studies. If the natural progression of disease is slowed by débridement chondroplasty despite the current level of collateral damaged induced, this may justify the collateral damage caused by the treatments currently in use. If not, the benefits of symptom improvement may still be a worthwhile approach to disease burden based upon improving the feel and function of the joint, which can allow a more productive activity level for patients.
This study evaluated articular cartilage specimens demonstrating generalized surface fibrillation changes in patients undergoing joint replacement surgery. Although a small sample size, the results indicate that débridement chondroplasty can be performed for these lesions in a more precise manner than previously shown, preserving Superficial Zone characteristics, and without inducing necrosis or collateral damage at the treatment site when compared to ablation-based radiofrequency treatments. These results are obtained from a device which combines non-ablation radiofrequency energy delivery and gentle mechanical implement use, yielding an hybrid device bridging the efficacious aspects of the two main débridement chondroplasty techniques available today. Low-level energies are enabled by the protective housing that shields the active electrode from the convective forces of the treatment site (i.e. fluid flow during arthroscopy); whereas, ablation-based devices often need to increase their energy output to accommodate such forces because their electrodes are exposed and unprotected.
Ceruleau is part of a new group of radiofrequency devices that utilize non-ablative low-level radiofrequency energy that is delivered via a protected electrode geometry which inhibits electrode-to-tissue contact. The protective housing can be optimally designed as a mechanical implement that functions as an adjunct to energy delivery while the active electrode can be optimally designed for low-level energy delivery. Accordingly, the device provides a convenient method of tactile and visual feedback that is especially useful when modifying surface tissue morphology such as during débridement chondroplasty. Surgeons are able to employ the mechanical implement as a treatment lead to energy use allowing real-time visualization of the treated tissue during energy delivery. With ablation-based radiofrequency devices, the surgeon cannot see the tissue during energy delivery because of electrode-to-tissue contact requirements and can only receive visual cues once the device is removed from the actual treatment site. For the ablation-based devices, the surgical technique used in this study of moving the ablation electrodes back and forth across the tissue simulates in vivo use conditions and likely is responsible for the lower depth of necrosis and intermixed live/dead cells observed in this study when compared to others which have demonstrated even more significant cartilage damage [16, 21, 30]. It remains, however, that an inadvertent pause in movement of ablation-based devices during use can cause ablation electrode imprinting, a subset of the more extensive tissue damage observed with these devices. Because electrode-to-tissue contact cannot occur with Ceruleau, electrode imprinting is not a tissue risk, allowing the surgeon a safer and more precise control of energy delivery.
The radiofrequency systems studied demonstrate different methods of delivering radiofrequency energy to tissues. Glider and Paragon deliver ablation-based energy. These cartilage ablation treatments are a necrosis-based intervention causing collateral damage via direct electrode-to-tissue contact similar to cancer cell ablation and cardiac arrhythmia foci ablation during which the electrical work is performed within target tissue located well below the tissue surface. Since the intended goal of débridement chondroplasty is a tissue preserving surface modification, it remains counter-intuitive to use a technology designed for sub-surface tissue treatment like radiofrequency ablation for disease states demanding tissue preserving surface modification. This concern is especially valid for a tissue type like articular cartilage which retains high water content and therefore can efficiently pool electro-thermal energy to an effectively detrimental level. Ablation-based devices have been the subject of numerous studies quantifying the degree of cartilage necrosis, not the lack of cartilage necrosis [9-22, 30, 31]. Consequently, cartilage researchers have looked for other additions to radiofrequency ablation energy delivery as mitigation for the effects caused by these devices [17, 32-34] since the residual tissue left behind at the treatment site is of poor quality, exhibiting significant tissue necrosis and collateral damage. This study further confirms the ablation-based iatrogenic injury to articular cartilage that has been repeatedly noted by others and, more specifically, demonstrates significant injury to and loss of the Superficial Zone which has been strongly associated with the progression of cartilage disease [15, 35-39].
Glider is an ablation-based monopolar system delivering radiofrequency energy directly to tissue much like that of standard electrocautery. The device tip design requires complete contact of the active electrode to the tissue during treatment, necessitating that the electrical current pass directly through the entire tissue thickness, creating damage which mimics electrical injury [40]. In this study, Glider eliminated the fibrillated cartilage of the lesions, but did not accomplish the goal of smoothing the articular surface. The treatment resulted in significant loss of normal cartilage tissue at the treatment site well below the level of original diseased tissue and into the initially intact Transitional Zone. The treatment replaced the fibrillated cartilage with a significant region of tissue loss and a resultant bed of necrotic cartilage. These results confirm other studies showing significant iatrogenic damage and loss of cartilage with monopolar ablation systems [10-12, 15, 16, 18, 19, 21, 30, 31]. Despite this evidence, some have put forth the notion that the iatrogenic creation of charred, necrotic articular cartilage tissue is useful to protect the underlying undamaged cartilage from excess energy deposition which normally occurs during the treatment [41]. It would seem, however, that creating iatrogenic damage to protect against further iatrogenic damage is counterproductive to cartilage treatment efforts, especially when no clear benefit from the treatment has been demonstrated. In fact, it is likely that such treatment may accelerate the natural progression and disease burden of osteoarthritis [15, 19, 21, 22, 35, 39]. Since this study demonstrated live cells within and around the area of fibrillated cartilage in the control specimens as a baseline, the extent of tissue loss and corruption of the Superficial and Transitional Zones caused by this treatment approach remains detrimental to the preservation of healthy cartilage tissue.
Paragon is an ablation-based bipolar device that also delivers energy directly to tissue via electrode-to-tissue contact. The device design requires significantly more power input to achieve the same amount of ablation energy delivery to the treatment site as that of Glider (Table 1). Consistent with other studies of bipolar energy delivery to articular cartilage [8, 9, 11, 14, 16, 18, 30], a distinct yet widespread geographic layer of necrosis and collateral damage was induced deep to the Transitional Zone. Significant cartilage loss was slightly less than that demonstrated with the Glider despite the much larger amount of energy required to effect bipolar cartilage ablation. This finding is partly due to the device design which requires high-level radiofrequency energy delivery to ablate tissue but allows the electrode to shunt high-level radiofrequency energy into the conductive media simultaneous with tissue ablation. Indeed, higher intra-articular fluid temperatures have been seen with ablation-based bipolar devices due to the high energy foundation required (i.e. plasma formation), a phenomenon which itself can lead to iatrogenic complications [34, 42, 43]. It remains unclear whether the gelatinization of cartilage at the treatment site observed in this study is limited to the area of induced chondrocyte necrosis in the Superficial and Transitional Zones or whether additional matrix or chondron collateral damage progresses deeper into the Transition Zone matrix as noted with Glider's monopolar energy delivery mechanism.
Ceruleau is a non-ablation bipolar device that is driven by a monopolar output which allows for a low-level and more precise amount of energy to be delivered to the treatment site. Since electrode-to-tissue contact cannot occur, Ceruleau delivers energy into the interfacing fluid medium within the protective housing. Therefore, the energy requirements for work are small as noted by the 25 Watts of energy deployed in this study to achieve a desirable outcome. The protective housing can be shaped to deliver this energy in a more precise manner than ablation-based devices which are dependent upon exposed tissue-contacting electrodes and are more subject to the varying conditions of the treatment site like arthroscopy fluid flow. Treatment energy requirements are further decreased since the system does not need to address impedance fluctuations originating from electrode-to-tissue contact which remains as a fundamental rationale for the high energy delivery requirements of the ablation-based systems to induce a tissue effect. For the surgeon, the low-level energy requirements of Ceruleau also translate to low bubble production which augments the ability to draw upon visual cues of the treatment site to control energy delivery. This study further demonstrates that ablation-based radiofrequency devices, because of their design, need to deliver much higher energy levels to articular cartilage than what is required for actual débridement chondroplasty.
The morphologic and histologic treatment results created by Ceruleau are vastly superior to those of other débridement chondroplasty techniques. The low-level energy delivery is configured to modify/precondition diseased cartilage to a state amenable to a safe and effective gentle mechanical débridement. The non-ablative energy affects the surface matrix structure of damaged cartilage tissue preferentially rather than the intact chondron and matrix tissue below the surface level as in ablation-based treatments. In this manner, the non-ablative energy takes advantage of the altered pericellular and extracellular matrices of diseased cartilage [44, 45] by preparing fibrillated tissue for débridement through augmented and/or naturally facile tissue cleavage patterns, creating a matrix-failure-based intervention. With the Ceruleau power wave shifted towards higher voltage levels rather than toward the higher current levels as required in ablation-based devices, the potential fields generated by Ceruleau allow beneficial electromagnetic affects as a precursor to additional tissue treatments. Others researchers have evaluated some aspects of non-contact ablation-level radiofrequency energy delivery to articular cartilage [16, 24, 46-48], noting the validity of such approaches. Since Superficial Zone characteristics can be retained for the lesions examined in this study, it may be possible to address the natural progression of disease as an early surgical intervention, rather than contributing to the loss of functioning articular cartilage during débridement chondroplasty. Further studies are required to assess the significance of the surface changes of the Ceruleau post-treatment specimens, particularly the increased density of live cells noted near the articular surface and within the Superficial Zone.
If iatrogenic cartilage damage can be eliminated during débridement chondroplasty, the procedure itself may be extended to other therapeutic interventions. With some ablation-based chondroplasty techniques, healing responses of the remaining cartilage have been observed [48, 49], indicating the possibility of in situ alteration of chondrocyte function in response to radiofrequency energy deposition. Dead chondrocytes at the treatment site as demonstrated with Glider and Paragon cannot be enrolled in additional therapeutic treatment efforts designed to augment tissue healing responses and will likely impair those efforts. If chondrocytes immediately surrounding the treatment site survive the precise removal of damaged tissue as observed in the Ceruleau treated specimens, the opportunity to effect beneficial changes in chondrocyte function remains whereby resident cell function may be recruited. For instance, chondrocyte transplantation procedures often fail due to impaired implant integration secondary to non-viable chondrocytes at the margins of the treatment defect [25, 50]. A more successful débridement chondroplasty or site preparation procedure may preserve chondrocyte differentiation potential evident in the Superficial and Transitional Zones [51] and may increase the chance of cellular-based integration. Preserving functioning Superficial Zone cartilage tissue will become even more important if the induction of chondrocyte healing responses becomes a standard treatment along with in situ cartilage repair or regeneration. Since ablation-based radiofrequency treatments significantly corrupt both the Superficial and Transitional Zones when present at the disease locale, precisely the zonal phenotypes that are deemed important for treatment of cartilage defects [51-55], and their use on articular cartilage should be further limited.
CONCLUSION
The mechanism of radiofrequency energy deposition upon tissues is particularly important in treatment settings requiring tissue preservation. Systems that delivered ablation-based energies caused tissue necrosis and collateral damage at the treatment site including corruption of cartilage Superficial and Transitional Zones; whereas, the non-ablation system created a smooth articular surface with Superficial Zone maintenance and without chondrocyte death or tissue necrosis. Ablation-based device systems can cause a worsened state of articular cartilage from that of pretreatment. Non-ablation energy can be successful in modifying/preconditioning tissue during débridement chondroplasty without causing collateral damage. Utilizing a non-ablation radiofrequency system provides the ability to perform successful débridement chondroplasty as an early surgical intervention for articular cartilage disease based upon matrix failure without causing additional articular cartilage tissue damage and may allow for other cartilage intervention success.
ACKNOWLEDGEMENTS
The work was performed at the Center for Integrated Nanotechnologies, United States Department of Energy, Office of Basic Energy Sciences User Facility, Los Alamos National Laboratory, Los Alamos, New Mexico (Contract DE-AC52-06NA25396) and Sandia National Laboratories (Contract DE-AC04-94AL85000) and Physicians Medical Center, Santa Fe, New Mexico. This study was supported by the New Mexico Small Business Grant Program WNM700, RO31, Los Alamos National Laboratory, Los Alamos, New Mexico and by NuOrtho Surgical, Inc., Fall River, Massachusetts.


