All published articles of this journal are available on ScienceDirect.
Alendronate Inhibits VEGF Expression in Growth Plate Chondrocytes by Acting on the Mevalonate Pathway
Abstract
Bisphosphonates decrease chondrocyte turnover at the growth plate and impact bone growth. Likewise vascular endothelial growth factor (VEGF) plays an important role in endochondral bone elongation by influencing chondrocyte turnover at the growth plate. To investigate whether the action of bisphosphonate on the growth plate works through VEGF, VEGF protein expression and isoform transcription in endochondral chondrocytes isolated from growing mice and treated with a clinically used bisphosphonate, alendronate, were assessed. Alendronate at 10µM and 100µM concentrations decreased secreted VEGF protein expression but not cell associated protein. Bisphosphonates are known to inhibit the mevalonate intracellular signaling pathway used by VEGF. Addition of the mevalonate pathway intermediates farnesol (FOH) and geranylgeraniol (GGOH) interacted with the low concentration of alendronate to further decrease secreted VEGF protein whereas FOH partially restored VEGF protein secretion when combined with the high alendronate. Similar to the protein data, the addition of alendronate decreased VEGF mRNA isoforms. VEGF mRNA levels were rescued by the GGOH mevalonate pathway intermediate at the low alendronate dose whereas neither intermediate consistently restored the VEGF mRNA levels at the high alendronate dose. Thus, the bisphophonate alendronate impairs growth plate chondrocyte turnover by down-regulating the secreted forms of VEGF mRNA and protein by inhibiting the mevalonate pathway.
INTRODUCTION
Bisphosphonate drugs inhibit osteoclastic resorption and therefore are used to treat bone disease characterized by increased osteoclast action [1, 2]. Bisphosphonates are used also to regulate circulating vascular endothelial growth factor (VEGF) concentrations within the body as a prophylactic against cancer metastasis [3].
A major function of VEGF is to modulate endochondral chondrocyte turnover during bone growth and development [4]. VEGF is synthesized from several isoforms each exerting differential effects on endochondral ossification [5]. The mature murine VEGF protein is translated from three primary transcripts, VEGF 120, VEGF 164, and VEGF 188 [6], generated by post-transcriptional exon splicing giving rise to protein isoforms that differ in extra cellular matrix binding affinities. The protein product of VEGF 120 is soluble with low binding affinity for extracellular matrix. In contrast, the VEGF 164 protein product is partially soluble but also binds to extracellular matrix while VEGF 188 is not soluble and binds only to matrix proteoglycans.
Because angiogenesis is a key element to osteogenesis, impairment of vascular invasion alters bone elongation. Reduced vascular invasion at the chondro-osseous junction has been observed with bisphosphonate treatment [7] corroborating the generalized decrease of VEGF and vascular expansion observed in bisphosphonate treated cancer patients. Nitrogen containing bisphosphonates such as alendronate inhibit the mevalonate pathway that produces isoprenoid lipids essential for the cell membrane anchoring of small GTP associated proteins (e.g., Ras, Rac, Rho) used in intracellular molecular signaling pathways [8]. Bisphosphonate treatment spanning the growth phase of juvenile patients may impact the events controlling longitudinal bone growth doing so by altering VEGF expression as a consequence of disrupting the mevalonate pathway.
In this study, the effect of a nitrogen containing bisphosphonate, alendronate, on VEGF transcript isoform expression and VEGF protein production in endochondral chondrocytes was assessed. Further, by providing mevalonate pathway intermediates, the question of how alendronate exerted its action on VEGF could be investigated. Characterizing the response of VEGF to bisphosphonates may provide insight on the impact of therapeutic bisphosphonate treatment on cartilage to bone conversion.
METHODS
Cell Culture
All experimental protocols used in this research were reviewed and approved by the institutional animal care and use committee of the University of California, Davis. Primary chondrocytes were isolated from costochondral growth plates of 15 stock inbred C57BL/6J male mice aged 28 days (Jackson Laboratories, Bar Harbor, ME, USA) as previously detailed [9, 10]. The chondrocyte preparation was, based on cellular phenotype and previous confirmation with density gradients [10], 50% hypertrophic, 40% proliferative, and 10% resting cell composition with trypan blue exclusion exceeding 95%. Chondrocytes were plated in DMEM/F12 + 10% FBS in 24 well microplates (Corning COSTAR, Corning, NY, USA) at a density of approximately 100,000 cells per ml (2 ml per well) and allowed to recover for 48 h.
After the initial 48h, media for the primary chondrocytes were replaced with DMEM/F12 + ITS media (BD biosciences, Bedford, MA, USA) containing 1% FBS. ITS media supplements low serum media and maintains endochondral chondrocyte growth in culture [11]. A key regulator of endochondral bone maturation is insulin like growth factor-I (IGF-I) [12] known to preferentially activate the p42/p44 MAPK pathway in proliferating growth plate chondrocytes by utilizing GTP associated proteins Raf and Ras [13]. IGF-I also regulates VEGF expression [13]. Thus all media were supplemented with 100ng/ml IGF-I (Peprotech Inc., Rocky Hill, NJ, USA) to ensure activation of the mevalonate pathway and expression of VEGF.
Triplicate chondrocyte cultures were incubated with 10µM or 100µM of alendronate either alone or in the presence of mevalonate pathway intermediates, 10µM farnesol (FOH) or 10µM geranylgeraniol (GGOH). The alendronate concentrations were selected based on concentrations experienced by patients and those used in culture experiments: estimated cellular concentrations experienced by patients receiving bisphosphonates for osteoporosis are 10µM or less [14] while cultured canine fibroblasts and osteosarcoma cells at a wide range of alendronate concentrations (1000 to 0.001µM) did not impair growth or viability [15]. FOH (Alfa Aesar, Ward Hill, MA, USA) and GGOH (ICN pharmaceuticals, Aurora, OH) are metabolized to farnesylpyrophosphate and geranylgeranyl-pyrophosphate, essential for the posttranslational modifi-cations necessary for membrane localization and activation of small G-proteins. The 10 µM concentration of mevalonate intermediates was based on published literature demonstrating that this level had no independent effect on bone [16]. Cells were incubated with the compounds for 24 h prior to collection for VEGF mRNA analysis. The entire experiment of 15 animals was fully replicated for treatment groups of six independent cultures.
mRNA Analysis
Total RNA was extracted and reverse transcribed for real-time PCR of the target VEGF-A gene transcripts (VEGF120, VEGF164, VEGF188) using a Taqman assay as previously described [10]. The Taqman systems were designed to be splice-variant specific by placing the reverse primer over the alternatively spliced exon [10]. The amplified PCR products were designed to be approximately 150 base pairs in length. Primer sequences were identified by analyzing sequences in GenBank using Vector NTI 9.1 and NCBI BLAST software. A universal forward primer was used 5’-GGAGAGATGAGCTTCCTACAGCA-3’ for all isoforms. The reverse primer was isoform specific: 5’-CCTCGGCTTGTCACATTTTTCT-3’ (VEGF 120); 5’-GCT CACAGTGATTTTCTGGCTT-3’ (VEGF 164); 5’-TCCTC GAACTGATTTTTTTTCTGG-3’ (VEGF 188). The Taqman probe was 5’-GATGTGAATGCAGACCAAAGAAAGACA GG-3’. The amplicons for each isoform were validated by agarose gel electrophoresis. To identify the most appropriate endogenous control, five commonly used housekeeping genes were assessed: a TaqMan PCR system recognizing 18S rRNA (ssrRNA), GAPDH (glyceraldehyde 3-phosphate dehydrogenase), HPRT1 (hypoxanthine phosphoribosyltrans-ferase 1), B2M (beta 2 microglobulin), and TFR2 (transferrin receptor 2; CD71). The murine HPRT1 was selected and used as the endogenous control because it was the most stably expressed gene for these chondrocytes. Final quantitation was done by the comparative CT method (User Bulletin no. 2, Applied Biosystems) and is reported as relative transcript level relative to a calibrator cDNA. Briefly, the signal of the endogenous HPRT1 control was used to normalize the target gene signals of each sample. The difference in the CT for the target and the CT for the internal control, termed ΔCT, was calibrated against the control value for cells treated only with ITS plus IGF-I. The relative linear amount of target molecules relative to the calibrator, was calculated by 2-ΔΔCt; thus, target gene transcript levels are expressed as an n-fold difference relative to the calibrator level from cells treated with ITS plus IGF-I.
Protein Analysis
Additional triplicate culture wells were treated and the media and cell lysates from each treatment group were collected 72 h after treatment administration for VEGF protein determination. Cells in each well were lysed in 0.1% triton-x100 in PBS with the lysates stored at -20 °C; prior to assay, lysates were centrifuged and the supernatants collected. Total protein content was assayed in media and cell lysates using the Bradford colorimetric method. Secreted VEGF protein in media and that associated with the cells were quantified by the commercially available enzyme-linked immunosorbent assay according to the manufacturer’s directions (Quantikine murine VEGF, R & D Systems, Minneapolis, MN, USA) and adjusted for total protein. Again, the entire experiment was fully replicated.
Statistics
VEGF protein expression and transcript isoform levels across treatments were analyzed by least-squares analysis of variance procedures using PROC GLM of SAS (version 8.0, 2001). Post hoc analyses were done using a Bonferroni adjustment. All experiments were replicated fully with the experimental replications yielding similar patterns of VEGF transcript and protein expression.
RESULTS
VEGF Isoforms
Growth plate chondrocytes treated with the low dose of alendronate (10µM) exhibited a 37% decrease in VEGF 120 and VEGF 164 isoform levels whereas the VEGF 188 isoform level was unchanged (Fig. 1; p < 0.01). The high alendronate dose (100 µM) similarly reduced VEGF 120 and VEGF 164 isoform levels by 32-36% but also reduced the VEGF 188 isoform level to the same extent (Fig. 1; p < 0.01).
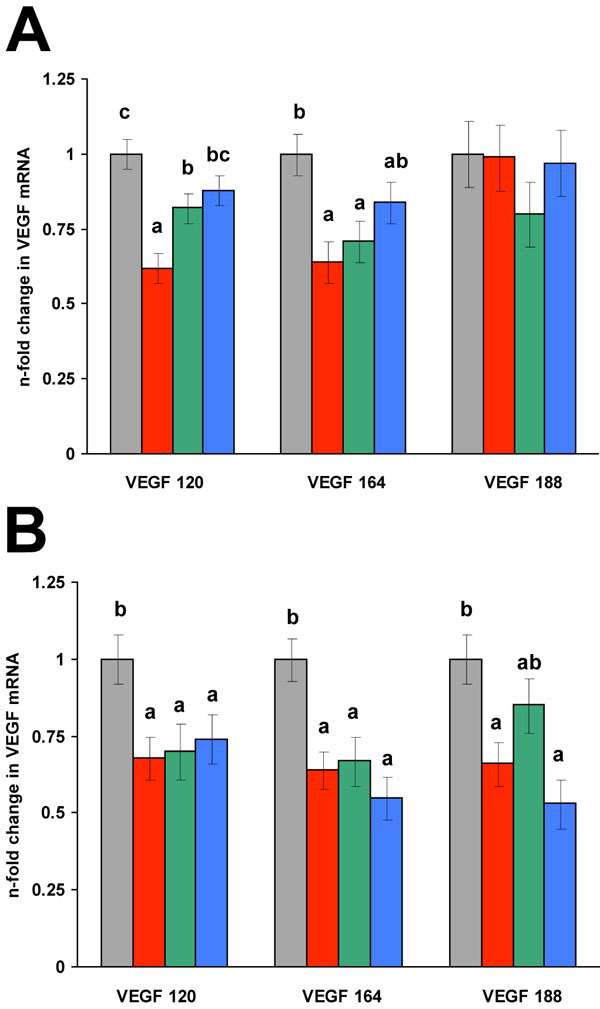
VEGF transcript isoform changes in endochondral chondrocytes treated with 10 µM (A) or 100 µM (B) alendronate for 24 hours. VEGF n-fold transcription changes for each isoform were calibrated relative to ITS +IGF-I control. Gray bars are ITS+IGF-I control, red bars are alendronate treated cells, green bars are alendronate plus 10µM FOH, and blue bars are alendronate plus 10µM GGOH. All values are expressed as least square means ± SEM. Within an individual VEGF isoform, bars labeled with different letters are significantly different from one another (p < 0.01).
Both VEGF 120 and VEGF 164 transcript levels underwent a partial to complete rescue with the addition of GGOH in combination with the low alendronate dose, whereas the addition of FOH only partially restored the VEGF 120 isoform level, but not the 164 transcript. Co-treatment of the cells with both FOH and GGOH in the presence of the low alendronate dose yielded the same results as cells treated with GGOH alone (data not shown). At the high dose of alendronate, the mevalonate intermediates were minimally effective at restoring the VEGF transcript levels with only the levels of VEGF 188 partially restored. In contrast to the results seen for the low alendronate dose, only the addition of FOH modestly restored VEGF 188 levels; neither FOH nor GGOH restored the 120 or 164 isoform levels. Provision of FOH and GGOH in combination with the high dose of alendronate was not different than either compound alone (data not shown).
VEGF Protein
Total VEGF secreted protein, adjusted for total protein, was decreased 32% and 50% by treatment with 10µM or 100µM alendronate, respectively (p < 0.001) (Table 1). The decrease in VEGF secreted protein induced by the alendronate at the low concentration was not restored to control levels by the addition of FOH and GGOH. Rather, addition of FOH to the 10µM dose of alendronate significantly decreased VEGF secreted protein beyond that observed for alendronate alone, reaching levels comparable to that caused by 100µM alendronate. The decrease in VEGF secreted protein observed in the 100µM dose was nearly restored by FOH but not by GGOH. This corresponded to the rescue seen for the VEGF isoform levels with the addition of FOH at the higher alendronate dose.
VEGF Protein (pg/ml), Adjusted for Total Protein, Secreted into the Media or Cell Associated from Murine Endochondral Chondrocytes Treated with Alendronate (ALN) and Mevalonate Intermediates Farnesol (FOH) and Geranylgeraniol (GGOH) for 72 h
| Media VEGF Protein | Control | ALN | ALN + FOH | ALN + GGOH |
|---|---|---|---|---|
| 10µM ALN | 306.9 ± 4.6a | 207.2 ± 4.6b | 132.0 ± 4.6c | 196.3 ± 4.6b |
| 100µM ALN | 279.5 ± 4.6a | 137.8 ± 4.6c | 239.4 ± 4.6b | 105.7 ± 4.6c |
| Cell VEGF Protein | ||||
| 10µM ALN | 19.5 ± 1.9a | 34.8 ± 1.9b | 26.4 ± 1.9a | 36.4 ± 1.9b |
| 100µM ALN | 18.6 ± 1.9a | 19.4 ± 1.9a | 9.7 ± 1.9b | 12.4 ± 1.9b |
Values expressed as LSMEANS ± SEM. Different superscripts within ALN dose (row) indicate means differ (p < 0.001); n = 6 for each different treatment group within a row.
Cell associated VEGF was increased by the 10 µM low alendronate dose and the addition of the mevalonate pathway intermediates did not further augment those levels. In contrast, the 100µM high alendronate dose did not alter cell associated protein but addition of either FOH or GGOH reduced VEGF levels, similar to the secreted VEGF response for the low alendronate dose.
DISCUSSION
Nitrogen containing bisphosphonate drugs are the primary non-surgical treatment for decreasing osteoclast action in various bone diseases such as Paget’s disease, osteoporosis and osteogenesis imperfecta. Bisphosphonates appear to improve tendon repair and prosthetic bone surgeries [17, 18] possibly by reducing apoptosis of osteoblasts and osteocytes [19]. Further, bisphosphonates have been suggested as a preventative for osteoarthritis [20]. Bisphosphonate drugs are also used extensively to reduce circulating VEGF thereby reducing metastasis for many forms of cancer and ameliorate bone pain associated with bone cancers [3, 19, 21-24]. Because VEGF is important in growth plate chondrocyte turnover and survival [4, 25], inhibition of VEGF by prolonged bisphosphonate treatment for bone disease or cancer may perturb growth plate chondrocyte turnover in juvenile patients.
In the present study, alendronate reduced VEGF transcript isoform levels and protein expression in endochondral chondrocytes by ~ 40%. Such a degree of inhibition is likely to have significant biological effects as similar reductions achieved by siRNA evoke physiological consequences [for example, reference 26]. The VEGF 120 isoform is the main secreted protein form of VEGF with VEGF 164 encoding both a secreted and a membrane bound protein and VEGF 188 strictly being membrane bound [27]. In the growth plate of juvenile mice the most abundant VEGF isoform is the VEGF 120 transcript representing 76.3% of all VEGF mRNA transcripts, whereas VEGF 164 and 188 represent 15.3% and 8.4%, respectively [10]. Treatment of chondrocytes with the low alendronate dose, the dose most similar to clinical studies, preferentially decreased the two predominant isoforms responsible for secreted protein: VEGF 120 and VEGF 164 without altering VEGF188 mRNA used for the bound form. The individual isoforms have distinct roles in angiogenesis and differential regulation of VEGF isoform expression has been reported for many systems [for example, references 28, 29]. A preferential decrease in the secreted VEGF forms by alendronate corresponds to the reduced vascular invasion at the chondro-osseous junction as seen in vivo following treatment with another bisphosphonate, pamidronate [30].
The main mechanism by which nitrogen bisphosphonates alter cellular function is through inhibition of various enzymes of the mevalonate pathway of isoprenoid lipid synthesis [31]. The isoprenoid intermediates farnesyl pyrophosphate and geranylgeranyl pyrophosphate are essential for the posttranslational modifications necessary for membrane anchoring and activation of the small GTP associated proteins involved in intracellular signaling. Provision of mevalonate pathway intermediates farnesol (FOH) and geranylgeraniol (GGOH) have been shown to rescue the effects of bisphosphonate mediated inhibition on cellular signaling [8, 32-34] thereby providing a mechanism to demonstrate whether the alendronate utilized the mevalonate pathway in the inhibition of secreted VEGF in chondrocytes.
Transcript levels of VEGF could be restored at the low alendronate concentration with the addition of GGOH indicating that transcription was regulated by intracellular signaling pathways utilizing geranylgeranylated proteins. The levels of VEGF 120 and 164 were increased only modestly with the addition of FOH. Though alendronate is reported to preferentially alter farnesyl pyrophosphate synthase [35, 36] the present study clearly demonstrates that low alendronate affects geranylgeranylation in cartilage. At the low alendronate dose, reflective of the concentrations experienced by patients receiving bisphosphonates [14], the present data suggest that geranylgeranylation is the preferentially inhibited step in bisphophonate treatment within growth plate chondrocytes.
The differential response to the mevalonate pathway intermediates is similar to findings of alendronate treated tumor and bone cells [16, 34]. VEGF mRNA expression is known to be regulated by Ras, a farnesylated G protein [37] though recent studies suggest that geranylgeranylated Rho may also be involved in regulating VEGF expression [38]. The data presented here suggest that at low alendronate concentrations, geranylgeranylated proteins such as Rho are involved in the synthesis of the mRNA forms responsible for secreted VEGF. Osteoclasts also rely predominantly upon geranylgeranylated proteins for formation and enzymatic activity [19] suggesting a coordinate regulation of vascular invasion and cartilage resorption at the chondro-osseous junction.
Low alendronate treatment alone and when combined with mevalonate pathway intermediates significantly reduced VEGF protein secreted into the media accompanied by an increase in cell associated VEGF. These data would suggest that alendronate altered the cellular content of both farnesylated and geranylgeranylated proteins important in the secretory process which in turn impaired release of the VEGF protein from the cell. By blocking isoprenoid modification, the alendronate may have further reduced the capacity of the chondrocyte to secrete VEGF beyond a reduction in transcription of the secreted form(s). Blocking secreted VEGF and inhibiting osteoclast function by reducing geranylgeranylated proteins by alendronate is consistent with the impaired vascular invasion and the reduced osteoclastic activity seen with bisphosphonates in vivo [30].
Vascular invasion is a necessary step in bone elongation and reducing that process would be expected to impact bone growth. However, reports of children with osteogenesis imperfecta given bisphosphonate therapy to reduce fractures do not describe negative impacts on bone growth [39]. It is possible that some of the G proteins affected by bisphosphonate treatment promote proliferation while others are involved in signaling pathways driving differentiation within the growth plate [40]. If alendronate at a clinically relevant dose primarily alters geranylgeranylation, it may only impair vascular invasion without negatively affecting prenylation steps involved in chondrocyte proliferation. Thus, the activity of the growth plate might not be significantly reduced, especially in the clinical condition of osteogenesis imperfecta where collagen is impaired. In contrast, normal rodents given bisphosphonates show significantly reduced bone length [7, 30] implying that VEGF inhibition has significant consequences on bone elongation corroborating the data derived from the VEGF knockout mice [5]. This is an important consideration for juvenile patients receiving chronic bisphosphonate treatment.
CONCLUSION
Alendronate, a nitrogen containing bisphosphonate, reduced secreted VEGF at the mRNA, protein, and secretory levels in growth plate chondrocytes indicating that bisphosphonates inhibit cellular turnover at the chondroosseous junction by reducing VEGF induced vascular invasion. This study showed that alendronate acted through the mevalonate pathway, specifically the geranylgeranylation of small GTP associated proteins. Though mRNA isoform levels were restored with the provision of mevalonate pathway intermediates, VEGF protein was only partially restored suggesting additional non-GTP protein associated pathways may be involved in the bisphosphonate inhibition of secreted VEGF protein. Prolonged inhibition of vascular invasion at growth plates reduces bone length. Therefore, the benefits derived from bisphosphonate therapy need to be weighed against the impact on growth plate function in juvenile patients undergoing long-term treatment.
ACKNOWLEDGEMENTS
This work was supported by California Agricultural Experiment Station Funds (CA-D*-ASC-5256-AH). The authors thank Janelle Belanger for technical assistance in the execution of the studies and The Lucy Whittier Molecular & Diagnostic Core Facility at UC Davis for designing and executing the Taqman PCR assays.


