RESEARCH ARTICLE
Raloxifene: Mechanism of Action, Effects on Bone Tissue, and Applicability in Clinical Traumatology Practice
Jose R. Caeiro Rey*, 1, Eduardo Vaquero Cervino2, Maria Luz Rentero3, Emilio Calvo Crespo4, Angel Oteo Álvaro5, Marta Casillas3
Article Information
Identifiers and Pagination:
Year: 2009Volume: 3
First Page: 14
Last Page: 21
Publisher ID: TOORTHJ-3-14
DOI: 10.2174/1874325000903010014
Article History:
Received Date: 22/12/2008Revision Received Date: 5/1/2009
Acceptance Date: 14/1/2009
Electronic publication date: 20/2/2009
Collection year: 2009

open-access license: This is an open access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by-nc/3.0/), which permits unrestrictive use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Raloxifene, a member of the class of selective estrogen receptor modulators (SERM), reproduces the beneficial effects of estrogens on the skeletal systems, without the negative effects estrogens on breast and endometrium.
This is a review article summarizing its mechanism, effects on bone and its applicability in traumatology clinical practice. In postmenopausal osteoporosis, this drug has been proven to decrease accelerated bone turnover, increase bone mineral density (BMD), and to structurally recover bone, decreasing the risk of vertebral fractures and the risk of non-vertebral fractures in patients with previous, severe vertebral fractures. Moreover, raloxifene appears to lower the risk of invasive breast cancer. Raloxifene would be efficacious in the prevention and treatment of postmenopausal osteoporosis.
We can therefore conclude that raloxifene would be efficacious in the prevention and treatment of postmenopausal osteoporosis, while reducing the risk of breast cancer when used at the indicated dose of 60 mg/day and with a low incidence of side effects.
INTRODUCTION
Postmenopausal osteoporosis is currently an important public health issue due to its widespread prevalence and the high socio-economic and healthcare impact it entails [1], which justifies the establishment of pharmacological and non-pharmacological measures aimed at treating this disease and at secondary prevention of the fractures associated with it [2].
One such pharmacological measure, hormone replacement therapy (HRT), initiated at the onset of menopause, has been demonstrated in numerous studies to be capable of improving menopause-related symptoms, while at the same time preventing the loss of bone mass associated with menopause [2-6]. HRT thereby manages to decrease the risk of osteoporotic vertebral and non-vertebral fractures in women over the age of 60 [7]. Nevertheless, due to its side effects, particularly relevant among which is the potential risk of developing breast cancer [8, 9], the use of this type of medication should not be contemplated as first line therapy for osteoporosis, reserving it instead for its current indication as treatment of perimenopausal symptoms and always for the shortest time possible [1].
These limitations of HRT stimulated the development of a series of non-hormonal compounds with a high affinity for estrogen receptors (ER) that are capable of reproducing the beneficial effects of estrogens on the skeletal system (estrogen agonist effect), without the negative effects on the breast and endometrium (estrogen antagonist effect), thanks to their selective binding to ER. These compounds were called Selective Estrogen Receptor Modulators or SERM [10-13].
Raloxifene, a second gereration benzothiophene-derived SERM (Fig. 1), has demonstrated this tissue specificity in humans and this dual action [14-16], making it an efficacious and safe drug in the prevention and treatment of postmenopausal osteoporosis [1].
 |
Fig. (1). Raloxifene’s chemical structure. |
In this article, we will review this drug’s mechanism of action, its selective effects on bone, and the determinants of bone strength, to then finish by analyzing its antifracture efficacy and applicability in clinical practice in traumatology.
MECHANISM OF ACTION
The classical model of estrogens’ mechanism of action is founded on the basis that estrogens, particularly estradiol (E2), must enter the nucleus of the target organ cells in order to bind there to a series of unoccupied, inactive proteins, called ER. These proteins, with two different isoforms, ERα (predominantly activating) and ERβ (which inhibits the former), transform into active receptors with a different spatial configuration (E2-ER) when they bind to E2, enabling them to simultaneously dimerize and subsequently interact with a specific sequence of DNA known as the Estrogen Responding Element (ERE) (Fig. 2, column A, level 2). A specific group of genes responsible for synthesizing the cell proteins that are in charge of triggering the estrogen effect on target reproductive tissues such as the uterus and the breast depend on ERE (Fig. 2, column A, level 3) [12-14].
 |
Fig. (2). Raloxifene’s mechanism of action. |
Moreover, there are two specific areas in the ER known as activation factors (AF); the first one, or AF-1, located at the site of interaction with the specific DNA sequence, and the second one, or AF-2, located at the site where the ligand binds. In order for the group of genes associated with the ERE to be activated and hence, for the proteins associated with them to be synthesized (gene expression), the E2 side-chain must interact with the AF-2 region (Fig. 2, column A, level 2) [12-14].
It is also known that ER do not have a single molecular binding site, but that they present two different domains, one for estrogen-type ligands and another one for antiestrogen-type ligands and SERM. Thus, depending on the binding site of the ligand to the ER, different spatial structures would be generated which would determine, at least in part, whether the ligand had a pure estrogen agonist action, a partial estrogen agonist action, or a pure estrogen antagonist action (Fig. 2, columns A and B, level 1) [12-14].
In light of all of the afore-mentioned, it is currently believed that ER do not act in the same way in all target tissues and that, in all likelihood, their action depends on whether the alpha or beta ER subtype is predominant in the tissue in question, on the nature of the ligand that binds to them (estrogen, antiestrogen, or SERM), on the cell transcription machinery (ERE and AF), and on the presence or absence of “helper” or regulating proteins (HP) [12].
Although this could account for how SERM perform different functions depending on the target tissue in question (pure agonist when interaction with ERα is predominant and pure antagonist interaction with ERβ is predominant), the precise mechanism by which raloxifene acts is not fully understood as yet.
On the one hand, we know that in order for it to exert its effects, this medication, like E2, must cross the cytoplasmic membrane and the nuclear membrane in order to bind to the ER present in the nucleus. There, the benzothiophene ring of the raloxifene molecule binds to the ER with an affinity similar to that of E2. This bond, in addition to preventing access of E2 to the ER, also brings about a change in the spatial configuration of the ER in charge of activating it and binding it to the ERE (Fig. 2, column B, level 2). However, unlike the intimacy with which this ring adapts to the ER, the basic side-chain of the molecule, which is large and inflexible, does not completely bind to it, making it “protrude” from the receptor area (Helix 12). This peculiarity provokes an alteration in the orientation of the molecule that blocks its possible interaction with AF-2, thereby preventing the associated genes from being activated and their gene transcription. In the opinion of some authors, this blockade would be the key to the estrogen antagonist effects of the drug on uterine and breast tissues [15, 16].
However, in contrast, in bone and in other non-reproductive tissues, raloxifene bound to the ER (RLX-ER) and with the help of a series of HP (activating, helping, and/or adapting proteins) would activate a specific sequence of DNA known as the Raloxifene Responding Element (RRE). A group of genes that bring about the synthesis of specific cell proteins that are responsible for the estrogen agonist effect of the drug on these non-reproductive tissues would depend on this element. Thus, it would be possible to explain how raloxifene imitates the effect of estrogens on non-reproductive tissues such as bone (estrogen agonist effect) (Fig. 2, column B, level 3) [15, 16].
However, in addition to this effect, raloxifene has demonstrated that it exerts two other direct actions on bone tissue. The first, which depends on the activation of its binding to the ligand, appears to indicate that this drug is capable of decreasing osteoclastic resorptive activity by up to 50%, interleukin-6 (IL-6) production, and up to 30% of the production of tumor necrosis factor α (TNF-α) at 6 months in vitro as well as in vivo [17-19]. Both of these latter substances constitute important mediators of bone resorption. The second, which depends in this case on the activation of the RRE, suggests that raloxifene is capable of increasing the production of transforming growth factor β3 (TGF-β3), thereby decreasing the number of osteoclasts as well as their resorptive activity (Fig. 2, column C) [20].
EFFECTS ON BONE AND DETERMINANTS OF BONE STRENGTH
The effects of raloxifene on bone and the determinants of bone strength (turnover, quantity, and quality) have been well studied in the last several years, not only thanks to numerous experimental works carried out in animals, but also based on the data derived from several clinical trials conducted with this drug in humans.
Effects on Bone Turnover
In postmenopausal osteoporosis, bone turnover is typically accelerated, whereby in each basic multicellular unit (BMU) a negative bone balance occurs as a result of bone resorption taking place at a faster rate than bone formation. Consequently, at present, this determinant of bone strength is considered to be the fundamental physiopathological cornerstone of the disease [21] and the process that must be controlled if bone biomechanical capacity is to be recovered.
In both ovariectomized animals [22-24] as well as in postmenopausal women with [25, 26] and without osteoporosis [27], raloxifene has demonstrated its ability to inhibit this accelerated bone resorption both short- and long-term, thereby increasing bone mineral density (BMD), preserving bone structure, and enhancing bone strength.
In the double-blind, placebo-controlled clinical trial conducted by Delmas et al. [27] in healthy postmenopausal women, raloxifene demonstrated that it significantly lowered bone turnover markers during the 24 months that the study lasted (bone-specific alkaline phosphatase by 15%, osteocalcin by 30%, and terminal carbonil fraction of the collagen type 1 C-telopeptide (CTX) by 40%).
Likewise, but this time in postmenopausal women with osteoporosis, raloxifene 60 mg/day demonstrated that it was capable of lowering the level of all bone turnover markers by 30 to 40% throughout the first year of treatment, a reduction that was maintained within premenopausal ranges during the entire time the studies lasted [25, 26]. The changes in the values of turnover markers (osteocalcin and creatinine-corrected urinary CTX) obtained in the study by Ettinger et al. [25] are presented in Fig. (3).
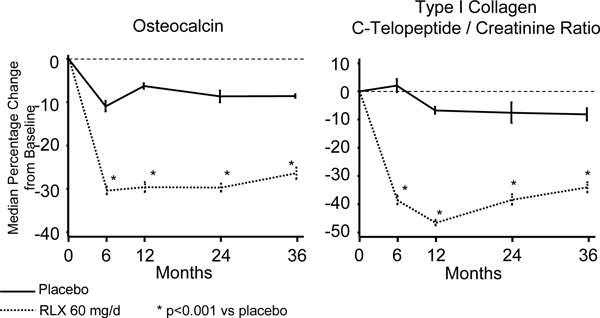 |
Fig. (3). Variation of bone turnover markers from baseline to endpoint from the MORE Study (36 months). |
In a recent study performed by Bjarnason et al. [28] in a subgroup of participants in the MORE Study (Multiple Outcomes of Raloxifene Evaluation), those patients with the most important reductions of bone formation markers (bone-specific alkaline phosphatase and osteocalcin) were precisely the ones who presented a greater decrease in the risk of vertebral fracture at the 3 year endpoint; no such correlation was seen with bone resorption markers (urinary CTX).
Effects on Bone Quantity (BMD: Bone Mineral Density)
Whereas women belonging to the control group were seen to loss bone mass, the group treated with raloxifene significantly increased BMD in the lumbar spine (2.5%), femoral neck (2%), and full body (1.9%) in the study conducted by Delmas et al. [27].
In the study performed by Ettinger et al. [25], after 36 months of treatment, the group treated with raloxifene at a dose of 60 mg/day, as well as the group that received 120 mg/day were both seen to have significantly increased BMD in both the lumbar spine (2.6% and 2.7%, respectively) and in the femoral neck (2.1% and 2.4%, respectively).
Cummings et al. [29, 30] reported that that improvement in spine BMD underestimated the degree to which antiresorptive drugs reduce the risk of vertebral fractures. Thus, the reduction in risk of fractures resulting from these antiresorptive treatments (which improve bone mineral density between 0% and 10%) was greater than predicted from the improvement in spine bone mineral density. This model estimates, for example, that antiresorptive treatments predicted to reduce the risk of fractures by 20% (RR = 0.80), based on improvement in spine bone mineral density, have been observed to reduce the risk of fractures by 45% (RR = 0.55). Similarly, the increase in BMD with other antirresorptive agents only account for 4% (calcitonin), 43% (estradiol), 16% with alendronate, and 7-28% with risedronate [31] of the decreases seen in the risk of vertebral fractures with mencioned drugs.
Sarkar et al. [32] starting with the data obtained from the MORE Study, examined BMD changes following 1 and 3 years of treatment with raloxifene 60 mg/day or 120 mg/day. The relation between baseline BMD and the baseline to endpoint changes in BMD with the risk of new vertebral fractures were analyzed in the different groups according to the dosis administered. As seen in other populations in the MORE Study, the women with the lowest baseline BMD in the lumbar spine and femoral neck were at greater risk for vertebral fracture. According the logistics regression model, only 4% of the reduction of the risk of vertebral fracture can be explain with the changes in BMD with raloxifene treatment and that there is as yet no explanation for the other 96% of the risk reduction. Authors conclude that the changes in BMD evaluated with raloxifene treatment are poor predictors of the decrease in risk of vertebral fracture.
These data corroborate prior observations that the effects of antirresorptive medication on BMD measures by means of dual energy X-Ray absorptiometry are too slight as to reflect the reductions observed in vertebral fractures [4], and suggest that other factors may also mediate in protection against fractures. In fact, the increase in bone turnover has been demonstrated to predict hip fractures in elderly women, regardless of BMD values [5].
Effects of Raloxifene on Bone Quality
From a qualitative perspective and by means of quantitative microrradiography of matched iliac creast biopsies taken at the initiation and after two years of treatment revealed that in patients participating in the MORE Study, both at a dosis of 60 mg/day as well as 120 mg/day, raloxifene was capable of increasing the mean degree of mineralization of bone (MDMB) (29% in the RLX 60 mg/day group, 8% in the raloxifene 120 mg/day group) with a greater rate of mineral heterogeneity in the treated group versus placebo. The MDMB profile observed in the biopsies following treatment with raloxifene is very similar to premenopausal physiological bone [26].
The effects of raloxifene on the histomorphometric variables were also determined by means of biopsies both prior to and after treatment in a six-month study in postmenopausal, Caucasian patients who received a daily dose of 60 mg of raloxifene or 0.625 mg of conjugated estrogens (CE). Twelve of the patients treated with raloxifene and 11 of the participants treated with CE underwent evaluation by bone biopsy during the baseline period and following six months of treatment. The primary efficacy parameters of bone formation/bone volume index and frequency of activation greatly decreased with treatment with conjugated estrogens, as well as with raloxifene, although the differences in the mean percentage change were not statistically significant between treatment groups. In both the women treated with raloxifene and in those treated with CE, no signs indicative of bone mineralization defects or plexiform bone or medullary fibrosis were seen [16].
In short and given the pure estrogen agonist effect on bone associated with the other two parallel inhibiting effects of the number and activity of osteoclasts, raloxifene is able to lower the rate of bone resorption in postmenopausal osteoporosis on the one hand and, on the other, it is capable of reversing the negative effects that this accelerated bone turnover has on bone resistance determinants (bone quantity and quality).
ANTIFRACTURE EFFICACY
Vertebral Fractures
In the MORE Study [25], Ettinger et al. analyzed the efficacy of raloxifene in reducing the risk of vertebral fractures in women with postmenopausal osteoporosis. This was a double-blind, randomized, multicenter clinical trial conducted in 7705 women with postmenopausal osteoporosis (femoral neck BMD T scores < 2.5) aged 31 to 80 years (mean age of 67 years). Results demonstrated that whereas 10.1% of the women in the control group (placebo + calcium and vitamin D) presented at least one new vertebral fracture, only 6.6% of the women treated with raloxifene 60 mg/day + calcium and vitamin D, and 5.4% of the women treated with raloxifene 120 mg/day + calcium and vitamin D presented said fractures. This meant that, for the treated group a significant reduction of the relative risk (RR) of suffering this type of fracture = 0.60 (95% Confidence Interval (CI) = 0.5-0.7, p < 0.01) with RR for raloxifene 60 mg/day = 0.7 (95% CI = 0.5-0.8) and RR for raloxifene 120 mg/day = 0.5 (95% CI = 0.4-0.7). This decrease in the risk of new vertebral fractures was equally significant in women with osteoporosis and previous vertebral fractures (RR reduction of 30%) as well as in women with osteoporosis who had not declared that type of fracture at the beginning of the study (RR reduction of 50%) [33].
In their re-analysis of the MORE Study data, Kanis et al. [34] evaluated the efficacy of raloxifene 60 mg/day in new vertebral fractures and new symptomatic fractures at three years in a subgroup of 3,204 postmenopausal women with osteopenia or osteoporosis without prior vertebral fractures. The relative risk of new vertebral fractures for the group of raloxifene treated patients compared to placebo was 0.53 (95% CI = 0.32-0.88) for the group of osteopenic patients and 0.31 (95% CI = 0.06-0.71) for those that had osteoporosis. None of the patients with osteoporosis who received raloxifene presented a new symptomatic vertebral fracture, whereas in the placebo-treated group, there were four new symptomatic vertebral fractures. There was no difference in raloxifene’s effect on vertebral fractures between osteopenic and osteoporotic patients (interaction p = 0.317 and 0.247, respectively). Authors concluded that 3 years of treatment with raloxifene 60 mg/day significantly lowers the risk of new radiographic vertebral fractures and of new symptomatic vertebral fractures in postmenopausal women with low BMD and who have not had previous vertebral fractures.
In a post-hoc analisys of this study, Maricic et al. [35] established that in a mere 12 months of treatment, 60 mg/day of raloxifene is capable of reducing the risk of new clinical vertebral fractures in the general study population by up to 68% and by 66% in the subgroup of women who had already previously suffered a vertebral fracture, and who therefore were at greater risk for another fragility fracture.
These data indicate that with raloxifene, relatively low BMD increases are translated into significant reductions in the risk of vertebral fractures similar to those seen with other antirresorptive agents [29, 36].
Non-Vertebral Fractures
Although in the afore-mentioned MORE Study, no decrease in the incidence of non-vertebral fractures or hip fractures was observed in the raloxifene-treated group of patients, a post hoc analysis of the same study had showed a relationship between the severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures [36].
Acording this analysis, severity of vertebral fractures at baseline was a stronger predictor of nonvertebral fracture risk than low BMD. This finding does not diminish the importance of mild vertebral fractures at baseline, but severe prevalent vertebral fractures are probably indicative of more significant architectural deterioration of bone that should be clinically addressed. In the small subgroup of women with severe baseline vertebral fractures in the MORE cohort, raloxifene significantly decreased the risk of subsequent nonvertebral fractures. Nevertheless authors pointed out that, although this analysis is helpful in understanding the effect of raloxifene on vertebral and nonvertebral fractures, the present data do not allow for a direct comparison of nonvertebral fracture efficacy of raloxifene with bisphosphonates and do not imply that raloxifene is an alternative to bisphosphonates for reducing the risk of nonvertebral fractures, especially of the hip.
Effects on Other Tissues
Although it was designed to evaluate the antivertebral fracture efficacy of raloxifene, the MORE Study demonstrated that this SERM was capable of lowering the risk of breast cancer in postmenopausal women by up to 76% [37]. This effect of raloxifene, perhaps having to do with its capacity to inhibit the proliferation of the human breast cancer cell line, MCF-7 [38], cannot a priori be extrapolated to young women with risk factors for breast cancer. The clinical trial STAR (Study of Tamoxifen and Raloxifen) that compared the efficacy of tamoxifene and raloxifene in women at high risk for breast cancer, concluded that raloxifene is as efficacious as tamoxifene in reducing the risk of invasive breast cancer and has a lower risk of venous thromboembolism and cataracts, albeit after eight years of follow-up, there was no statistically significant difference as regards the risk of non-invasive breast cancer [39]. Likewise, in the same study the risk of developing another type of cancer, fractures, ischemic disease, or suffering a cerebrovascular accident was similar for both drugs. In the study carried out by Cummings et al. [37] previously referred to, raloxifene did not increase the risk of uterine cancer.
Several studies have demonstrated the effects of SERM on lipid metabolism. Specifically, it appears that raloxifene is capable of lowering LDL-cholesterol levels without there being a concomitant increase in the level of triglycerides, while the concentration of HDL-cholesterol increases by up to 15% althought there was no observed effect on end-event from lowering cholesterol [36, 40].
Side Effects
In general, raloxifene’s safety profile exhibits a very low incidence of adverse side effects [41, 42]. Raloxifene raises the risk of venous thromboembolism from 1.5- to 3-fold [25], although in general, the frequency of superficial venous thrombophlebitis was less than 1%. Among these adverse effects, the most noteworthy is a 7% increase in hot flashes in the initial posmenopausal period [43]. Another adverse reaction that has been observed is leg cramps (5.5% with raloxifene and 1.9% with placebo in the prevention studies and 9.2% with raloxifene and 6.0% with placebo in treatment studies).
Long-term safety was analyzed by Martino et al. [44, 45] who carried out a study to evaluate raloxifene’s safety after 8 years of treatment in 4,011 postmenopausal women with osteoporosis who had been included in the CORE Study (Continuing Outcomes Relevant to Evista), a continuation of the MORE Study. The incidence of hot flashes, leg cramps, and peripheral edema, adverse events associated with treatment with raloxifene, was not statistically significant between the different treatment groups (p > 0.5 for each event). Over the course of the 8 years, hot flashes and leg cramps were reported more frequently in the raloxifene-treated group than in the placebo group (p < 0.001, p = 0.008, respectively), albeit such was not the case with peripheral edema (p = 0.24). The relative risk of thromboembolism in the group of patients who received raloxifene (2.9 events per 1,000 women-years) compared to the placebo-treated group (1.3 events per 1,000 women-years) was 2.17 (95% CI = 0.83 to 5.70). The incidence of venous thromboembolic events was 2.2 and 1.3 events per 1,000 women-years in the raloxifene and placebo groups, respectively.
Cardiovascular risks were assesed in RUTH (Raloxifene Use for the Heart Trial) study [46]. This was a randomized, double-blind, placebo-controlled, international study conducted in postmenopausal women at risk for major coronary events. A total of 10,101 postmenopausal women with established coronary heart disease or at increased risk for coronary heart disease were randomly assigned to either placebo (N = 5057) or raloxifene 60 mg/day (N = 5044). Raloxifene treatment neither increased nor decreased the risk of coronary events (533 versus 553 events; HR = 0.95; 95% CI = 0.84-1.07). There were no significant between-group differences in the incidences of stroke, death from any cause, or overall death from cardiovascular causes. Women treated with raloxifene had higher risks of venous thromboembolic events (absolute risk increase, 1.2 per 1000 woman-years) and fatal stroke (absolute risk increase, 0.7 per 1000 woman-years), but had lower risks of clinical vertebral, but not nonvertebral, fractures (absolute risk reduction, 1.3 per 1000 woman-years) and death from noncardiovascular causes (absolute risk reduction, 1.7 per 1000 woman-years). Significantly fewer women in the raloxifene group had one or more hospitalizations for any cause (52% vs 54%; HR = 0.91; 95% CI = 0.87-0.96; P = 0.001).
Insofar as genitourinary safety is concerned, raloxifene did not cause endometrial hypertrophy nor increased the risk vaginal bleeding [27, 36]. In fact, in the clinical trials in which raloxifene (n = 317) was compared to continuous combined hormone replacement therapy (n = 110) or with cyclic hormone replacement therapy (n = 205), the incidence of breast symptoms and uterine bleeding in the women treated with raloxifene was significantly lower than in the women treated with either of the two hormone replacement therapy (HRT) regimens.
Raloxifene should not be administered to pregnant women or to men, since its innocuousness has not been demonstrated in these patients.
APPLICABILITY IN CLINICAL TRAUMATOLOGY PRACTICE
Raloxifene had demonstrated a decreasing the risk of vertebral fractures in patients with postmenopausal osteoporosis [25], due to its ability of slowing the accelerated bone turnover to premenopausal ranges; increase BMD, structurally recover bone, and increase bone strength. In a post-hoc analysis, authors pointed out that in the subgroup of women with severe baseline vertebral fractures, raloxifene significantly decreased the risk of subsequent nonvertebral fractures [36]. Likewise, raloxifene is the first antirresorptive agent to demonstrate a decrease in the risk of vertebral fractures in patients with osteopenia [35]. According all these data, raloxifene is a clearly indicated for the prevention and treatment of postmenopausal osteoporosis, especially in those cases with vertebral predominancy.
In addition to having demonstrated its antifracture effect, raloxifene presents beneficial effects on lipid metabolism and breast tissue. It is capable of lowering LDL-cholesterol levels and increasing HDL-cholesterol ones [36, 40] and it also appears to reduce the risk of invasive breast cancer [37, 39]. Both effects increase the value of this drug, making it especially useful in women with osteopenia or postmenopausal osteoporosis with lipid alterations or breast cancer risk factors.
Consequently, at a dose of 60 mg/day (that is, one tablet per day) taken at any time of the day, independently of meals, and associated with calcium (1200-1500 mg/day) and vitamin D3 (400 UI/day) (especially in women with a diet that is low in calcium), raloxifene is efficacious in the prevention and treatment of postmenopausal osteoporosis, above all in predominantly vertebral osteoporosis, while at the same time, presenting a low incidence of side effects and exhibiting a beneficial effect on breast tissue by decreasing the risk of breast cancer. These characteristics make raloxifene a pharmacolocial alternative in our arsenal of treatments that would help us to treat our patients with osteoporosis.
CONFLICT OF INTEREST
Maria Luz Rentero and Marta Casillas are full-time employees of Lilly. All other authors declared no conflict of interest.
REFERENCES
| [1] | Muñoz-Torres M, Alonso G, Mezquita RP. Prevención y tratamiento de la osteoporosis Endocrinol Nutr 2003; 50: 1-7. |
| [2] | Brown JP, Josse RG. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada Can Med Assoc J 2002; 167(Suppl 10): S1-S34. |
| [3] | Stevenson JC, Baum M. Hormone replacement therapy. Should be used selectively BMJ 1994; 309(6948): 191-2. |
| [4] | Hillard TC, Whitcroft SJ, Marsh MS, et al. Long-term effects of transdermal and oral hormone replacement therapy on postmeno-pausal bone loss Osteoporos Int 1994; 4(6): 341-8. |
| [5] | Kanis JA, Stevenson JC. Effect of estrogen therapy on bone density in elderly women N Engl J Med 1994; 330(10): 715-6. author reply |
| [6] | Felson DT, Zhang Y, Hannan MT, Kiel DP, Wilson PW, Anderson JJ. The effect of postmenopausal estrogen therapy on bone density in elderly women N Engl J Med 1993; 329(16): 1141-6. |
| [7] | Landa MC. Papel de la terapia hormonal sustitutiva, en la prevención y tratamiento de la osteoporosis menopáusica An Sist Sanit Navar 2003; 26(Suppl 3): 99-105. |
| [8] | Chlebowski RT, Hendrix SL, Langer RD, et al. WHI Investigators Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women the Women's Health Initiative Randomized Trial JAMA 2003; 289(24): 3243-53. |
| [9] | Beral V. Million Women Study Collaborators Breast cancer and hormone-replacement therepy in the Million Women Study Lancet 2003; 362: 419-27. |
| [10] | Kauffman RF, Bryant HU. Selective estrogen receptor modulators Drug News Perspect 1995; 8(9): 531-9. |
| [11] | Palacios S. Moduladores selectivos of the receptores estrogenicos Rev Clin Esp 1999; 670-74. |
| [12] | Riggs BL, Hartmann LC. Selective estrogen-receptor modulators Mechanisms of action and application to clinical practice N Engl J Med 2003; 348(12): 618-29. |
| [13] | Petersen NM, Briggs AL. Selective estrogen receptor modulators Clin Rev Bone Miner Metab 2005; 3(1): 19-30. |
| [14] | Zanchetta JR, Talbot JR. Raloxifeno In: Osteoporsis. ED. Buenos Aires (Argentina): Ed, Panamericana 2001; pp. 359-65. |
| [15] | Grese TA, Sluka JP, Bryant HU, et al. Benzopyran selective estrogen receptor modulators (SERMS): Pharmacological effects and structural correlation with raloxifene Bioorg Med Chem Lett 1996; 6: 903-08. |
| [16] | Bryant HU, Glasebrook AL, Yang NN, Sato M. A pharmacologic review of raloxifene J Bone Miner Metab 1996; 14: 1-9. |
| [17] | Jilka RL, Hangoc G, Girasole G, et al. Increased osteoclast development after estrogen loss: Medication by interleukin-6 Science 1992; 257: 88-91. |
| [18] | Yang NN, Venugopalan M, Hardikar S, Glasebrook A. Identification of an estrogen response element activated by metabolites of β-estradiol and raloxifene Science 1996; 273(5279): 1222-25. |
| [19] | Gianni W, Ricci A, Gazzaniga P, et al. Raloxifene modulates inter-leukin-6 and tumor necrosis factor-{alpha} synthesis in vivo: Re-sults from a pilot clinical study J Clin Endocrinol Metab 2004; 89(12): 6097-99. |
| [20] | Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor Cell 1987; 51: 941-51. |
| [21] | Heaney RP. Is the paradigm shifting? Bone 2003; 33: 457-65. |
| [22] | Turner CH, Sato M, Bryant HU. Raloxifene preserves bone strength and bone mass in ovariectomized rats Endocrinology 1994; 135(5): 2001-5. |
| [23] | Evans GL, Bryant HU, Magee DE, et al. Raloxifene inhibits bone turnover and prevents further cancellous bone loss in adult ovariec-tomized rats with established osteopenia Endocrinology 1996; 137(10): 4139-4. |
| [24] | Jerome CP, Lees CJ. Raloxifene increases bone mass and reduces bone turnover in ovariectomized cynomolgus monkeys J Bone Miner Res 1996; 11: 445-1. |
| [25] | Ettinger B, Black DM, Mitlak BH, et al. For the multiple outcomes of raloxifene evaluation (MORE) Investigators. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial JAMA 1999; 282(7): 637-45. |
| [26] | Lufkin EG, Whitaker MD, Nickelsen T, et al. Treatment of estab-lished postmenopausal osteoporosis with raloxifene: a randomized trial J Bone Miner Res 1998; 13: 1747-54. |
| [27] | Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of Raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women N Engl J Med 1997; 337(23): 1641-47. |
| [28] | Bjarnason NH, Sarkar S, Duong T. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in post-menopausal osteoporosis Osteoporos Int 2001; 12: 922-30. |
| [29] | Sarkar S, Mitlak BH, Wong M, et al. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy J Bone Miner Res 2002; 17(1): 1-10. |
| [30] | Cummings SR, Black DM, Thompson DE, et al. Effect of alendro-nate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial JAMA 1998; 280(24): 2077-82. |
| [31] | Cummings SR, Karpf DB, Harris F, et al. Improvement in spine bone density and reduction in risk of vertebral fractures during treatment with antirresorptive drugs Am J Med 2002; 112: 281-9. |
| [32] | Watts NB, Cooper C, Lindsay R, et al. R elationship between changes in bone mineral density and vertebral fracture risk associated with risedronate J Clin Densitom 2004; 7: 255-61. |
| [33] | Gluck O, Maricic M. Raloxifene: Recent information on skeletal and non-skeletal effects Curr Opin Reumatol 2002; 14: 429-32. |
| [34] | Kanis JA, Johnell O, Black DM, et al. Effect of raloxifene on the risk of new vertebral fracture in postmenopausal women with osteopenia or osteoporosis: A reanalysis of the multiple outcomes of raloxifene evaluation trial Bone 2003; 33(3): 293-300. |
| [35] | Maricic M, Adachi JD, Sarkar S, Wu W, Wong M, Harper KD. Early effects of raloxifene on clinical vertebral fractures at 12 months in postmenopausal women with osteoporosis Arch Intern Med 2002; 162(10): 1140-3. |
| [36] | Delmas P, Genant HK, Crans G, et al. Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial Bone 2003; 33(4): 522-32. |
| [37] | Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial JAMA 1999; 281: 2189-97. |
| [38] | Sato M, Glasebrook AL, Bryant HU. Raloxifene: a selective estrogen receptor modulator J Bone Miner Metab 1994; 12(S2): S9-S20. |
| [39] | Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes The NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 Trial JAMA 2006; 295: 2727-41. |
| [40] | Bass KM, Newschaffer CJ, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women Arch Intern Med 1993; 153: 2209-16. |
| [41] | Evista- U.S. Food and Drug Administration [online]. Available from URL:http://www.fda.gov/cder/foi/label/2007/022042lbl.pdf [Accessed 2007, Sep 28]; |
| [42] | Evista-EU Summary of Product Characteristics Available from URL: http://emea.europa.eu/humandocs/PDFs/EPAR/Evista/H-184-PI-en.pdf [Accessed 2007 Sep 28]; |
| [43] | Johnston CC Jr, Bjarnason NH, Cohen FJ, et al. Long-term effects of raloxifene on bone mineral density, bone turnover, and serum lipid levels in early postmenopausal women: three-year data from 2 double-blind, randomized, placebo-controlled trials Arch Intern Med 2000; 160: 3444-50. |
| [44] | Martino S, Cauley JA, Barrett-Connor E, et al. Continuing Outcomes Relevant Evista®: incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene J Natl Cancer Inst 2004; 96: 1751-61. |
| [45] | Martino S, Disch D, Dowsett SA, et al. Safety assessement of raloxifene over eight years in a clinical trial setting Curr Med Res Opin 2005; 21(9): 1441-52. |
| [46] | Barrett-Connor E, Mosca L, Collins P, et al. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women N Engl J Med 2006; 355: 125-37. |







