All published articles of this journal are available on ScienceDirect.
Polyethylene Oxidation in Total Hip Arthroplasty: Evolution and New Advances
Abstract
Ultra-high molecular weight polyethylene (UHMWPE) remains the gold standard acetabular bearing material for hip arthroplasty. Its successful performance has shown consistent results and survivorship in total hip replacement (THR) above 85% after 15 years, with different patients, surgeons, or designs.
As THR results have been challenged by wear, oxidation, and liner fracture, relevant research on the material properties in the past decade has led to the development and clinical introduction of highly crosslinked polyethylenes (HXLPE). More stress on the bearing (more active, overweighted, younger patients), and more variability in the implantation technique in different small and large Hospitals may further compromise the clinical performance for many patients. The long-term in vivo performance of these materials remains to be proven. Clinical and retrieval studies after more than 5 years of in vivo use with HXLPE in THR are reviewed and consistently show a substantial decrease in wear rate. Moreover, a second generation of improved polyethylenes is backed by in vitro data and awaits more clinical experience to confirm the experimental improvements. Also, new antioxidant, free radical scavengers, candidates and the reinforcement of polyethylene through composites are currently under basic research.
Oxidation of polyethylene is today significantly reduced by present formulations, and this forgiving, affordable, and wellknown material is still reliable to meet today’s higher requirements in total hip replacement.
INTRODUCTION
Polyethylene is a well-known material to orthopaedic surgeons since Sir John Charnley popularized it in his hip LFA (low friction arthroplasty) [1]. The rationale under the use of this polymer has been extensively reviewed [2], with clear advantages over other polymers used in early total hip arthroplasty (THA) designs, such as Teflon (polytetrafluor-ethylene, PTFE) and Delrin (polyacetal). Polyethylene and ultra high molecular weight polyethylene (UHMWPE), became the most popular and standard material in THA friction pairs since the 1960’s until the 1990’s. Early alternative solutions based on metal-on-metal (MOM) or ceramic-on-ceramic (COC) articulations showed significant pitfalls in many designs [3, 4]. The standardized solution of UHMWPE for the acetabulum was at the time inexpensively processed by machining components out of extruded UHMWPE bars, followed by gamma irradiation sterilization of large batchs to doses ranging from 25 to 40 KGy. It is worth observing that UHMWPE is a semicrystalline polymer constituted by a crystalline phase (the crystals, observed in the transmission electron microscopy –TEM- images as lamellae) and an amorphous phase (the disordered state) that allows some rearrangement of the crystals under mechanical stresses [5]. This characteristic semicrystalline structure of UHMWPE is illustrtated in Fig. (1). The material had a significantly large molecular weight (ultra-high), meaning long polymer chains that were further reinforced by covalent molecular bridges, also known as cross-links, originated upon gamma irradiation.
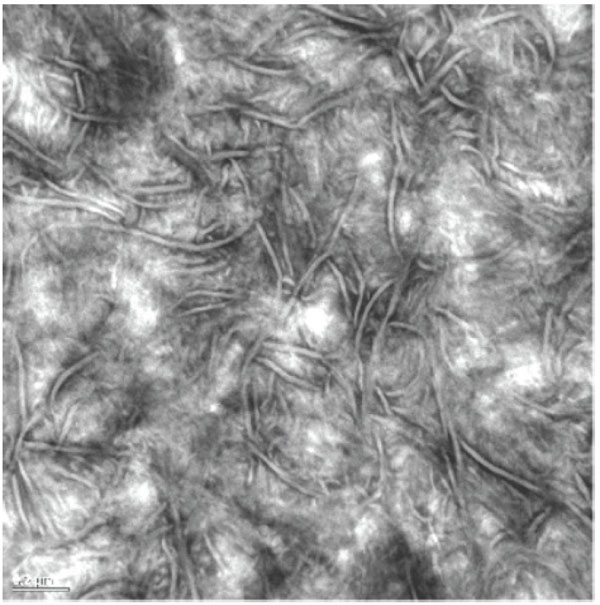
UHMWPE is a semicrystalline polymer with crystalline and amorphous regions. Crystals appear as ribbon-like lamellae and amorphous regions as gray areas in Transmission Electron Micrographs.
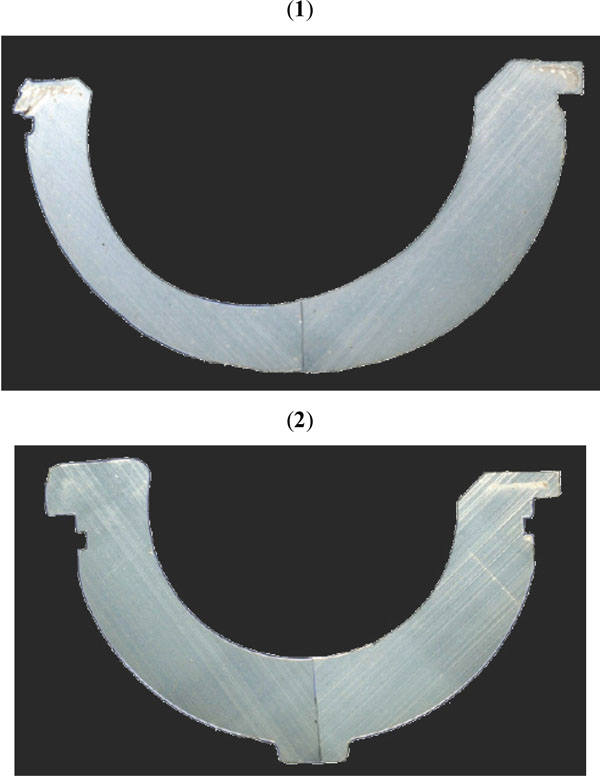
In the 1980’s and early 1990’s, long-term survivorship of polyethylene cups was compromised by osteolysis and aseptic loosening. After significant research, wear particle production was found the triggering mechanism. Cement particles were first considered as the causative agent, the so called “cement disease”, but when the osteolysis also prevailed in uncemented designs, polyethylene particles were apparent as the main pathogenetic factor, particularly those in the submicron range. Aseptic loosening and osteolysis resulted in significant concern about polyethylene quality and wear resistance in the Orthopaedic community, and extensive research was devoted to clarify what caused the polyethylene failure and how to prevent it. Unimplanted polyethylene components from the shelf were analyzed to investigate the material prior to in vivo use, and subsurface white bands of high density material (Fig. 2) were found in components with long shelf life [6, 7]. Retrieval studies of long-term failures of polyethylene cups showed that polyethylene was not homogeneous. Occasional subsurface white bands and fusion defects in the bulk material were frequently identified in acetabular polyethylene components. Further analysis to correlated these findings with wear and performance [8], but while consolidating defects were found more frequently in early-retrieved cups without affecting survivorship, subsurface defects were found responsible for material loss at the articulating surface. Fourier transformed infra red (FTIR) analysis of the material from the surface to the bulk [9] confirmed that oxidized polyethylene was the constituent of this white band defects. Mechanical analysis of this material showed a comparatively brittle behavior with respect to non-oxidized polyethylene, as well as distinct fracture modes [10].

After long shelf life (5 years) of a gamma irradiated in air conventional polyethylene component, a subsurface white band is clearly detected.
From these and other findings, it was concluded that oxidation of the polyethylene component concentrated in the subsurface in long shelf aged implants and was deleterious for the performance of the joint.
CAUSES AND AVOIDANCE OF OXIDATION IN CONVENTIONAL POLYETHYLENE
Oxidation of irradiated polyethylene is unavoidable as soon as the polymer is in contact with air or in vivo fluids. Eventually, several groups and particularly Costa et al. [9, 11] characterized the oxidation in failed and never implanted polyethylene components. The oxidation in never implanted components was more intense with longer times on the shelf before implantation [6, 7], and chemical studies confirmed that irradiation (gamma irradiation was the standard procedure of sterilization) in the presence of oxygen led to chain scission of the polyethylene long chain and free radical generation at the crystal surfaces [12]. In view of the semicrystalline structure of the material that determines many mechanical properties, any change in the microstructure may significantly alter the mechanical behavior of the material [5].
The oxidative degradation of the material progressed in the presence of oxygen after the irradiation process within the permeable package, while the component was sitting on the shelf. To eliminate oxygen out of the system is not an easy task, but the first reaction of the Orthopaedic community was to standardize barrier packaging to avoid oxygen permeability, and thus perform gamma irradiation sterilization either in vacuum or in the presence of inert gases (typically, nitrogen, and argon), and to recommend the avoidance of implantation 5 years after manufacture and irradiation [13]. Other non-penetrating sterilization methods, such as ethylene oxide or gas plasma, were reconsidered and reincorporated to the process, although ethylene oxide was originally discontinued because of the cumbersomeness of its method. Irradiation was discovered as a valuable technique not only for efficient sterilization, but also for crosslinking of the polyethylene chains.
Although the previous efforts generally succeeded in avoiding shelf aging, there has been growing evidence of the occurrence of in vivo oxidation of polyethylene components, not only after gamma sterilization in air, but also following gamma sterilization in nitrogen [13, 14]. Furthermore, in vivo oxidized polyethylene retrievals (Fig. 3) display a characteristic regional pattern, with regions protected by metal parts (bearing surface and backside) reaching lower oxidation than more exposed areas (rim) [13-16]. The unavoidable occurrence of in vivo oxidation in gamma sterilized polyethylene components stems from post-irradiation induced free radicals, which, upon oxygen availability, initiate the oxidation cycle and the associated physical changes.
After in vivo exposure, high oxidation areas are seen as a subsurface white band in cross-sections of a gamma air (1) and gamma inert (2) sterilized acetabular liner retrievals. Images courtesy of Professor Steven Kurtz (Implant Research Center. Drexel University. Philadelphia).
NEW POLYETHYLENES TO DECREASE WEAR
The development of first-generation highly crosslinked polyethylene formulations were intended to provide medical grade UHMWPEs with an extremely high wear resistance and good oxidative stability. Thus, high doses of gamma or electron beam radiation are employed to promote an elevated crosslink density (i.e. covalent bonds) into UHMWPE, which, in turn, is responsible for a notable increase in wear resistance. In first-generation highly crosslinked polyethylenes, two different approaches were adopted to achieve oxidation resistance. First, annealing, involved a single thermal treatment below the melting temperature of UHMWPE so that crystallinity and mechanical properties were preserved [17]. However, the commercial highly crosslinked polyethylene obtained by gamma irradiation, annealing and finally gamma inert sterilization contained residual free radicals with the potential to oxidize in vivo [15]. The second approach was based on post-irradiation remelting of the polymer above the crystalline transition. This strategy allowed for elimination of free radicals up to undetectable levels, but at the expense of crystallinity changes and diminished mechanical properties [17, 18].
IN VIVO AND RETRIEVAL STUDIES ON HXLPEs
From a wear perspective, radiographic and retrieval studies have confirmed a significantly reduced femoral head penetration for both annealed and remelted HXLPE in the first decade of implantation [19-30]. Table 1 offers a summary the significant papers. Not only clinical mid-term follow-up studies clearly show this wear rate decrease, but more precise methods such as roentgen stereogrammetry analysis (RSA) confirms this important finding in three-dimensional evaluation of cups in randomized studies (Table 2).
Summary of Significant Clinical Studies Confirming a Wear Rate Decrease with 1st Generation HXLPE in the Mid-Term Follow-Up
| Study | Design and Follow-up | HXLPE | HEAD | Follow-Up | Mean Wear Rate mm/yr (After Bedding-In) | Wear Rate Decrease |
|---|---|---|---|---|---|---|
| Dorr et al. JBJS A 2005 | Prospective, cohorts37 hips/37 | Durasul95 kGy, remelted | 28 mm CrCo | 5 years | 0.029 vs 0.065 | 45% |
| D’Antonio et al. CORR 2005 | Retrospective, comparative56 hips/53 | Crossfire105 KGy, annealed | 28 mm CrCo | Mean 5 yr (min 4) | 0.036 vs 0.131 | 72% |
| Engh et al. J Arthr 2006 | Prospective, randomized 208 hips | Marathon50 kGy, remelted | 28 mm CrCo | 5.7 yr(4.1-7.2) | 0.01 vs 0.19 | 95% |
| Olyslaegers et al. J Arthr 2008 | Case-control with historical60 hips/20 | Longevity100 KGy, remelted | 28 mm CrCo | XLPE 5.06 yr (52-69 mo),Std PE 5.1 yr (55-79) | 0.05 vs 0.101 | 50% |
| García-Rey et al. J BJS-B 2008 | Prospective, randomized45 hips/45 | Durasul95 kGy, remelted | 28 mm CrCo | 66.3 mo(60-92) | 0.006 vs 0.038 | 84.3% |
| Geerdink et al. CORR 2009 | Randomized, double blind17 hips/23 | Duration30 KGy,annealed | 28 mm CrCo | Mean 8yr(7-9) | 0.088 vs 0.142 | 38% |
Clinical Studies with 3D Analysis of Wear Rate with 1st Generation HXLPE
| Study | Design | HXLPE | Head | Follow-Up | Conclusions |
|---|---|---|---|---|---|
| Bragdon et al.J Arthrop 2007(EFORT ‘09) | Non-consecutive, non-randomized30 hips | Longevity 100 kGy, remelted | 28mmCrCo (16 hips)vs36mmCrCo(14 hips) | 3 yearsEFORT 09:7-10yr | No diff 3D between28 and 36mm |
| Röhrl et al.Acta Orthop 2007 | Retrospective, comparative56 hips/53 | Crossfire105 KGy, annealed | 28 mm CrCo | XLPE 6 yr, Std 5 yr | No wear rate progression at 6yr re oxidation |
| Digas et al.Acta Orthop 2007 (cem) | Prospective, randomized56 hips | Durasul95 kGy, remelted | 28 mm CrCo | 5 years | 0.001 vs0.06mm/yr (3D) (98% decrease) |
| Digas et al.Acta Orthop 2007 (hybr) | Prospective, randomized contralat control32 hips/32 | Longevity100 KGy, remelted | 28 mm CrCo | 5 years | 0.00 vs0.057mm/yr(3D)(99-100% decrease) |
| Glyn-Jones et al.J Arthrop 2008 | Prospective, doub-blind, rand, controlled26 hips/25 | Longevity100 KGy, remelted | 28 mm CrCo | 2 years | 0.06 mm/yr vs0.10 (3D)(40% decrease) |
Regarding the clinical failure modes, the revision rates of acetabular liners of both first-generation HXLPE formulations due to loosening, instability and infection remain comparable to those of conventional gamma inert sterilized liners after a decade of service [19]. Recent retrievals studies have confirmed that annealed highly crosslinked polyethylene acetabular liners oxidize in vivo (Fig. 3) and that, in some cases, exhibit damage at non-articulating areas [19, 30, 31]. Thus, delamination, subsurface cracking and even partial fracture of the rim have been observed under relatively unusual clinical circumstances, that is recurrent dislocations, trauma or edge loading [30]. Furthermore, according to a consecutive series of retrieved annealed HXLPE acetabular liners, the incidence of rim damage secondary to in vivo oxidation and mechanical loading appears to be as low as 5% [30]. Although remelted HXLPE acetabular liner retrievals exhibit near-zero oxidation levels after a decade of in vivo exposure, rapid crack initiation and rim fracture cases have also been reported because of the combination of decreased mechanical strength [32-34]. Nevertheless, the incidence of rim fracture in remelted retrievals appears to be as low as that of retrieved annealed liners [19]. Very recently, the hypothesized complete oxidative stability of remelted HXLPE components has been questioned in view of the increasing trend of oxidation with implantation time observed in retrievals and elevated oxidation measured after ex vivo aging studies [19, 35].
PRESENT AND FUTURE SOLUTIONS TO OXIDATION
The clinical performance of first-generation HXLPE will need further research to confirm the benefits of the reduction in femoral head penetration and the clinical relevance, if any, of in vivo oxidation and crack initiation in the long-term, during the second decade of implantation. Currently, second-generation HXLPEs represent a promising alternative to first-generation HXLPEs as they take advantage of alternative stabilization strategies, such as natural antioxidants (vitamin E), mechanical annealing, or sequential irradiation and annealing processes [36-41].
In the sequentially annealed material, experimental studies proved that 4.9 mm thickness maintains a similar wear rate than thicker components, thus confirming thin components are not disadvantageous under this formulation [42], and support larger heads without more damage near impingement [43].
As for the vitamin E stabilized material, different formulations are produced either when the antioxidant is blended with the polyethylene at the time of consolidation, or if vitamin E diffuses through the consolidated polymer in the mechanism of doping. A gradient distribution of antioxidant is typically associated with the diffusion method, but polyethylene oxidation is controlled by vitamin E, as shown after 36 months of artificial aging [44], and the post-irradiation oxidation decreases with increasing vitamin E concentrations [45]. On the other hand, the vitamin E blended formulation (< 0.1 % of vitamin E in weight) has been shown to maintain the mechanical properties of polyethylene [46].
Other antioxidant strategies are under development, using different free radical scavengers, such as nitroxide-TEMPO (2,2,6,6-Tetramethylpiperidine-1-oxyl) [47], HPAO (hindered phenol antioxidant) [48], or anthocyanin extracts [49]. Last but not least nanoscale modifications are also being studied to reinforce the polyethylene, namely composite reinforcement by multiwalled carbon nanotubes [50], or grafting with 2-methacryloyloxyethyl phosphorylcholine polymer [51] among others. Needless to say that the ongoing refinement into the polyethylene basic science and proposals will not stand until full developments are ready, and experimental and clinical data prove the concept and solidity of new polyethylene formulations. The oxidative resistance and mechanical performance of this last generation HXLPE are promising based on in vitro testing of the most advanced products, such as sequential annealing and vitamin E blended or diffused polyethylenes, but their impact in the clinical practice needs to be established.


