All published articles of this journal are available on ScienceDirect.
Intra-operative Clodronate Rinsing Improves the Integration of the Femoral Stem in a Prospective, Double-blinded, Randomized, Placebo-controlled Clinical RSA-study
Abstract
Background:
Periprosthetic bone loss after Total Hip Arthroplasty (THA), detected as an early migration of the prosthesis may predict later loosening of the implant.
Objective:
We hypothesized that intra-operative bisphosphonate rinsing would reduce bone resorption after THA. It might therefore be possible to achieve better early fixation of the implant.
Methods:
Nineteen patients suffering from arthrosis were recruited in a prospective, double-blinded, randomized, placebo-controlled clinical pilot trial. Patients were operated with an uncemented Bimetric stem using tantalum markers. The femoral proximal intramedullary canal was rinsed with 1mM clodronate in nine patients and with saline in 10 patients. These patients were followed for two years using radiostereometric analysis (RSA), dual energy x-ray absorptiometry (DXA) and the Harris Hip Score (HHS).
Results:
We did not found any significant differences between the study groups with regards to the primary output measures (maximum total point motion, MTPM). However, there was evidence that clodronate could affect periprosthetic bone quality; a beneficial effect in BMD in Gruen zone 3 during the two-year follow-up was observed, BMD decreased less in the clodronate group (p = 0.02). The maximal x-translation of the stem at 3-24 months was significantly two-fold, being higher in the placebo group (p = 0.02). The baseline BMD and the maximal total point motion (MTPM) at 3-24 months showed a positive correlation in the clodronate group and a negative correlation in the placebo group.
Conclusion:
In conclusion, further studies with larger patient groups and longer follow-up periods are needed to estimate the clinical importance of these findings and further to prove if an intraoperative clodronate rinsing prior to application of femoral stem during THA can prevent periprosthetic bone loss.
Clinical Trial Registration No.: NCT03803839
1. INTRODUCTION
Total Hip Arthroplasty (THA) is an effective and widely used method for treatment in end-stage osteoarthritis of the hip, providing pain relief and improved joint function. Periprosthetic bone loss after THA is a disturbing phenomenon which threatens osseointegration, the long-term integrity of the implant-bone interface, and may cause aseptic loosening of the prosthesis and periprosthetic fracture resulting in hip revision [1]. In addition, implant survival appears to be closely dependent on the initial integration of the prosthesis. Increased early migration of the prosthesis is an indicator for aseptic loosening and late implant failure [2, 3].
There appear to be three factors involved in the etiology of aseptic prosthesis loosening. First, oscillating fluid pressure at the bone-implant interface may lead to osteolysis [4-6]. Second, stress shielding may occur when the stem of the prosthesis disturbs normal biomechanical loading [1]. Third, the inflammatory process caused by wear debris around the prosthesis [7].
Bisphosphonates have a strong, long-lasting and well-established inhibitory effects on bone resorption. The inhibitory effect of bisphosphonate is cell-mediated, mainly by a direct inhibitory effect on osteoclasts [8-10]. Clodronate, an early bisphosphonate, is a non-amino compound, which in contrast to amino bisphosphonates, also has anti-inflammatory [11] and analgesic effects [12], making it favorable for the prevention of aseptic loosening after THA [13].
Previous short-term randomized controlled trials have shown that the use of bisphosphonates after primary THA prevents periprosthetic bone loss and thus enables a higher Bone Mineral Density (BMD), especially in the proximal femur [14-18]. Furthermore, three population-based cohort studies have reported bisphosphonate users, especially osteoporotic patients, to have as much as a 59% reduced risk for revision surgery [19-21]. There are few controlled trials focusing on the ability of bisphosphonates to reduce the early migration of prosthesis after THA. These reports have presented results both for and against the hypothesis that bisphosphonates may decrease the migration rate of prosthesis components after arthroplasty [17, 22-25]. Sköldenberg et al. found no effects on uncemented stem (n = 73) migration using EBRA-software during a 24 month period and during a four year period [24] after THA using 35 mg risedronate once a week for six months postoperatively [17]. Five days of 90 mg of pamindronate therapy after THA did not have an effect on cup migration (EBRA-software) at 2 years (n = 44) [25]. However, Schilcher et al. found a reduction of cemented acetabular component (n = 60) migration (RSA) during a 24 month follow-up period after THA with local intraoperative treatment with ibandronate, 3 mg in 3 mL saline [22]. Similar results were noted in another study (EBRA) using a single infusion (4 mg) of intravenous zoledronic acid after cementless THA with 50 patients, with an average follow-up period of 2.8 years [23].
Thus, the benefit of bisphosphonates in minimizing stem migration after THA is still not clear. The purpose of our study was to examine whether the local intraoperative administration of clodronate could reduce periprosthetic bone loss and stem migration after primary THA. To our knowledge, this is the first report on the effects of intraoperative intramedullar bisphosphonate rinsing on postoperative femoral stem stability after cementless THA using the Radiostereometric Analysis (RSA) method.
2. MATERIALS AND METHODS
2.1. Trial Design
In this prospective, double-blinded, randomized, placebo-controlled clinical trial (Level of Evidence II) with a two year follow-up period, we rinsed the proximal intramedullary cavity of the femur with either clodronate (60 mg clodronate in 1000 ml saline) or saline (1000 ml saline) prior to the application of the non-cemented Bimetric-femoral stem. All operations were done from the postero-lateral approach. Patients were randomized into two treatment groups; either the clodronate or a placebo group. The ethics committee of the University Hospital of Oulu (100/2000) approved the study protocol and patients provided their written informed consent. All patients, surgeons and other medical staff were blinded as to the patients’ study group. Only the pharmacists who prepared the rinsing solution were not blinded. They labeled the packages with the letters A or B, according to a randomization list made by the biostatistician. Randomization was done in groups of four. One arthroplasty surgeon from our study group attended all operations as an operating surgeon or as an assistant. The bone-implant interfaces were first carefully rinsed with pulsatile lavage using saline to remove all debris and blood from the site. Next, before installation of the prosthesis, the rinsing was performed again with pulsatile lavage using either the clodronate solution or with the plain saline solution according to the pouch received from the hospital pharmacy. The time for rinsing was about one minute. We have proved in an earlier experimental study that a 1 minute incubation time is sufficient for the almost total binding of bisphosphonate (clodronate) onto the bone surface and a 1 mM concentration causes a sufficient reduction in bone resorption and osteoclast apoptosis rate [9]. We chose clodronate because of its anti-inflammatory effects, our previous experience of it and its great cost-benefit ratio [13]. The patients received an uncemented total hip replacement with the Bimetric hip stem (Echo Bi-Metric, Biomet, USA). Full-weight bearing was allowed immediately as tolerated.
2.2. Study Population
The patients included in the study were recruited during the years 2004-2012 from the patients entering the hospital and suffering from arthrosis and for whom a primary THA was planned. Twenty patients were recruited for this pilot study, but one patient quit for personal reasons after the baseline measurements. Patients suffering from renal insufficiency, hypercalcemia, malignant tumors, osteoporosis and patients who were contemporaneously treated with another bisphosphonate or teripatatide or aminoglycoside were excluded from the study. In addition, only patients under 75 years (due to the average life expectancy of Finns, the entire follow-up period in our project was 10 years; this report contains results from the first 2 years of follow-up) were included in the study. Inclusion criteria was good bone quality; thick cortexes of the femur should be seen in the hip x-ray to enable the application of the non-cemented femoral stem. When recruiting the final ten patients of the planned thirty patients to the study, we had to include the size of the femoral intramedullary canal in the recruiting criteria as the size of the remaining tantalum-marked stems was limiting the study patient selection. In the end, we decided to limit the recruiting of study patients and to settle for twenty patients as we were unable to recruit patients suitable for the large-sized tantalum-marked stems. The study patients had no prosthesis infections or other complications that would have been a cause for interruption in the study.
2.3. Measurements and Analysis
RSA and dual energy x-rays absorptiometry (DXA) were carried out at a post-operative baseline within five days of surgery, and at 3, 6, 12 and 24 months with the Harris Hip Score (HHS) estimated at 3, 6, 12, and 24 months. Five tantalum markers of 0.8 mm were implanted into the greater trochanter and three markers in the lesser trochanter region, and three were installed in the stem. These specific Bimetric stems with tantalum markers were prepared. The markers implanted into the bone were stable and were used as references to detect any change in the position of the stem. The first RSA examination served as a reference for all further examinations; all evaluations were related to the position of the prosthesis relative to the bone at that time. Radiographs were taken using 2 fixed x-ray tubes (Fuji, FCR 5000) with the patient in the supine position. Two films were taken within two seconds at a 45-degree angle with a biplanar calibration cage, according to RSA standards [26, 27] (Fig. 1). RSA evaluation was performed using UmRSA software (version 6.0, RSA Biomedical Innovation, Umea, Sweden). The accuracy (up to 0.047 - 0.121 mm) and precision (0.03 mm) of RSA were very high. The mean error fitting upper limit was set to 0.25 mm, the upper limit for the condition number 90. The linear movements of the stem were analyzed as translations along 3 axes (x, y, z) and the angular movement was analyzed as rotations around 3 axes (x, y, z); Maximal Total Point Motion (MTPM) was calculated (the length of the translation vector of the point in a rigid body that has the greatest motion). RSA analyses were calculated between 0-24 months. We also defined the variable for maximal translation and Maximal Total Point Motion (MTPM) at 3-24 months, where the maximal migration between 3-6, 3-12 or 3-24 months was noted. It allowed a comparison for stem migration with one variable and to study stem migration after the expected integration of stem should have happened. It has been noted earlier that after implantation, it will take about 3 months for osseointegration [28].
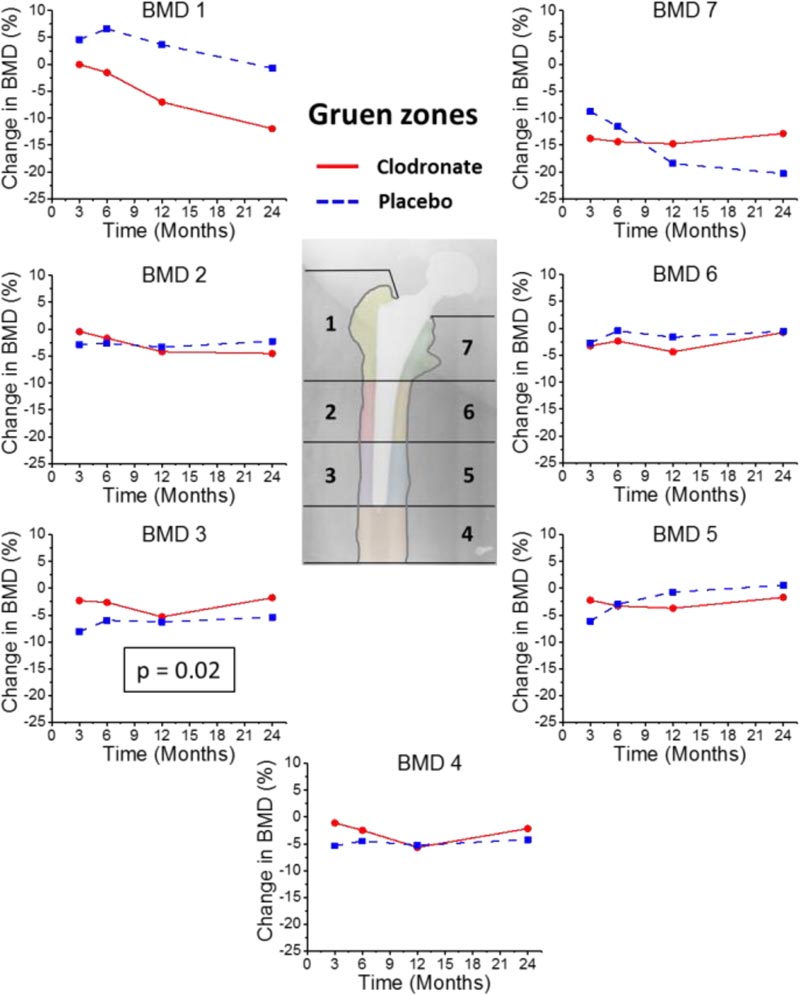
BMD was measured by dual-energy X-ray absorptiometry, DXA, on a scanner (Lunar Prodigy, GE Medical System). Two different X-ray beam energies enable bone attenuation to be separated from soft tissue attenuation. The output from the DXA examination includes an image of the proximal femur divided into 7 regions of interest based on the Gruen zones (Fig. 1) and quantitative data (BMD of each Gruen zone) [29]. The orthopedic metal removal software (enCore v17) was used for data analysis. The patient was made to lie on a scanner table in a supine position with both lower extremities secured in the neutral patella position. A leg positioning device was used to ensure constant positioning of each limb for every scan. The post-operative DXA measurement served as a control. The accuracy and precision errors of DXA were both < 1%. In clinical use, precision errors are < 5% [30]. We evaluated the differences in BMD between study groups in each Gruen zone during 0-24 months. We also analyzed differences between the intra-sample medial and lateral plateau between groups to calculate a summarized evaluation of BMD changes caused by stem possible translation: the average value of lateral Gruen zones 1-3 minus the medial Gruen zones 5-7 in each patient at each time point.
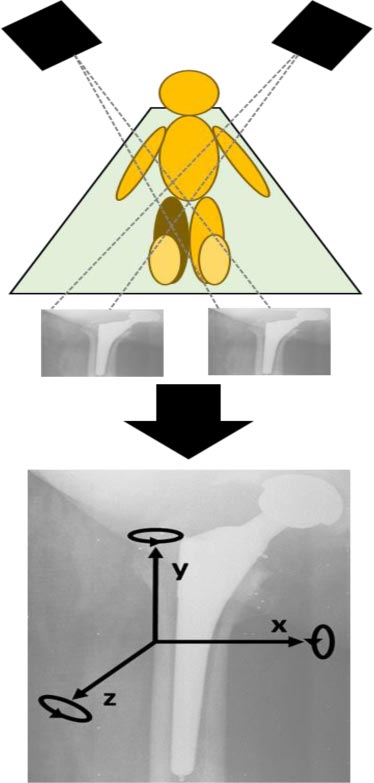
2.4. Outcomes
The primary outcome measure was MTPM during the 2 year follow-up period. The secondary outcome measure was BMD in 7 Gruen zones during the 2 year follow-up period. The other outcome measures were migration including x- (transverse), y- (longitudinal) and z- (sagittal) translations of the femoral stem during the 2 year follow-up period, the maximal MTPM at 3-24 months correlation between baseline BMD and maximal MTPM at 3-24 months and HHS during the 2 year follow-up period.
2.5. Statistical Analysis
Summary statistics are presented as means with Standard Deviation (SD) or as median with 25th-75th percentiles. For cross-sectional data, the Student’s t-test and Fisher’s exact test were used for between-group comparisons, the former for continuous data and the latter for categorical data. Spearman’s correlation coefficient (ρ) was calculated. Repeatedly measured data, i.e. MTPM, BMD and migration, were analyzed using a linear mixed model (LMM) with the patient as a random effect; the covariance-structure was chosen according to Akaike’s information criteria. Estimated between-group differences with 95% confidence intervals (95% CI) are presented according to LMM. As the LMM allows the analysis of unbalanced data sets without imputation, all available data were used in the analyses. Two-tailed p-values have been reported. Analyses were performed using SPSS (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) and SAS (version 9.3, SAS Institute Inc., Cary, NC, USA).
3. RESULTS
The patients’ mean age in the clodronate group (n = 9, 5 female) at surgery was 65.8 ± 8.5 years, and in the placebo group (n = 10, 4 female), it was 62.6 ± 5.3 years. The patients’ detailed characteristics are presented in Table 1. None of these patients had undergone a hip revision during the 24-month follow-up period for aseptic loosening. One patient in the placebo group underwent a small revision procedure, as a 36 mm metal head was installed by mistake instead of the 32 mm head. The revision was done two months after THA. The group of study patients had no prosthesis infections or other complications that would necessitate interrupting the study. Thus no adverse effects were reported to be caused by clodronate. In the RSA analyses in the follow-up from 0 to 24 months, there were 7 study patients in the clodronate group, after excluding 2 patients due to the RSA problems in baseline measurements. The RSA analyses from 3 to 24 months included all 9 patients. All 10 patients were included in the placebo group in both RSA analyses. In the DXA analyses, all but one patient`s data was included in the placebo group from 3 to 24 months. HHS was measured successfully from all patients in both the groups.
3.1. Effect of Clodronate Rinsing on the Peri-implant BMD
In both groups, the baseline BMD values were significantly lower in proximal areas, Gruen zones 1 and 7, compared to the distal areas of Gruen zones 2-6, p ≤ 0.001. There was a significant group and time interaction (p = 0.016) at 24-months of follow-up in the Gruen zone 3 only, BMD decreased less in the clodronate group than in the control group. No significant differences between the groups at single time points and other Gruen zones were noticed (Fig. 2), although the absolute BMD value was higher in the clodronate than in the placebo group in Gruen zones 3-7 (Table 2).
| - | Clodronate (n=9) | Placebo (n=10) |
|---|---|---|
| Age at surgery (years) | 65.8 ± 8.5 | 62.6 ± 5.3 |
| Sex (male/female) | 4/5 | 6/4 |
| Body mass index (kg/m2) | 26.1 ± 2.3 | 24.8 ± 3.3 |
| Primary diseases | 1 ± 1 | 2 ± 2 |
| Drinking (alcohol) | - | - |
| mild (men <14 and women <7 units/week) | 0 | 1 |
| severe (men ≥14 and women ≥7 units/week) | 2 | 1 |
| Smoking (tobacco) | - | - |
| mild (< 20 pack-years) | 1 | 0 |
| severe (≥ 20 pack-years) | 2 | 2 |
| Osteoarthritis grade (Kellgren-Lawrence) | - | - |
| KL0 | 0 | 0 |
| KL1 | 0 | 0 |
| KL2 | 1 | 2 |
| KL3 | 4 | 4 |
| KL4 | 4 | 4 |
| Heterotopic ossification | 2 | 1 |
| Side (right/left) | 4/5 | 5/5 |
| Bearing surfaces | 9 | 10 |
| metal-on-metal | 6 | 7 |
| ceramic-on-ceramic | 3 | 2 |
| metal-on-polyethylene | 0 | 1 |
| Harris hip score (0-100) | - | - |
| 3 months | 79 ± 21 | 76 ± 22 |
| 6 months | 87 ± 14 | 87 ± 17 |
| 12 months | 88 ± 15 | 92 ± 11 |
| 24 months | 93 ± 11 | 93 ± 11 |
| Variable | Time | Clodronate | Placebo | Difference | CI | |||
|---|---|---|---|---|---|---|---|---|
| - | Month | Mean | SD | Mean | SD | Estimate | Min | Max |
| BMD (Gruen zone 1) | 0 | 1.02 | 0.15 | 1.02 | 0.12 | 0.00 | -0.17 | 0.17 |
| BMD (Gruen zone 1) | 3 | 1.01 | 0.18 | 1.07 | 0.12 | -0.05 | -0.24 | 0.13 |
| BMD (Gruen zone 1) | 6 | 1.01 | 0.22 | 1.08 | 0.13 | -0.07 | -0.27 | 0.13 |
| BMD (Gruen zone 1) | 12 | 0.95 | 0.21 | 1.03 | 0.17 | -0.07 | -0.27 | 0.14 |
| BMD (Gruen zone 1) | 24 | 0.89 | 0.14 | 1.00 | 0.17 | -0.08 | -0.26 | 0.08 |
| BMD (Gruen zone 1) | total | - | - | - | - | -0.06 | -0.23 | 0.12 |
| BMD (Gruen zone 2) | 0 | 2.01 | 0.22 | 2.03 | 0.22 | -0.02 | -0.29 | 0.25 |
| BMD (Gruen zone 2) | 3 | 2.01 | 0.26 | 1.98 | 0.27 | 0.02 | -0.25 | 0.29 |
| BMD (Gruen zone 2) | 6 | 1.98 | 0.29 | 1.99 | 0.32 | 0.00 | -0.27 | 0.27 |
| BMD (Gruen zone 2) | 12 | 1.90 | 0.27 | 1.95 | 0.30 | -0.01 | -0.29 | 0.26 |
| BMD (Gruen zone 2) | 24 | 1.91 | 0.23 | 1.99 | 0.33 | -0.02 | -0.39 | 0.26 |
| BMD (Gruen zone 2) | total | - | - | - | - | -0.01 | -0.27 | 0.25 |
| BMD (Gruen zone 3) | 0 | 2.21 | 0.25 | 2.21 | 0.20 | 0.01 | 0.25 | 0.24 |
| BMD (Gruen zone 3) | 3 | 2.21 | 0.28 | 2.04 | 0.26 | 0.13 | -0.11 | 0.38 |
| BMD (Gruen zone 3) | 6 | 2.15 | 0.29 | 2.08 | 0.19 | 0.07 | -0.17 | 0.31 |
| BMD (Gruen zone 3) | 12 | 2.04 | 0.18 | 2.08 | 0.22 | 0.02 | -0.23 | 0.27 |
| BMD (Gruen zone 3) | 24 | 2.10 | 0.16 | 2.10 | 0.24 | 0.08 | -0.16 | 0.33 |
| BMD (Gruen zone 3) | total | - | - | - | - | 0.06 | -0.18 | 0.30 |
| BMD (Gruen zone 4) | 0 | 1.94 | 0.35 | 1.95 | 0.22 | -0.02 | -0.31 | 0.28 |
| BMD (Gruen zone 4) | 3 | 1.96 | 0.31 | 1.85 | 0.23 | 0.06 | -0.23 | 0.36 |
| BMD (Gruen zone 4) | 6 | 1.89 | 0.33 | 1.87 | 0.24 | 0.02 | -0.27 | 0.31 |
| BMD (Gruen zone 4) | 12 | 1.77 | 0.32 | 1.85 | 0.26 | -0.03 | -0.32 | 0.26 |
| BMD (Gruen zone 4) | 24 | 1.88 | 0.26 | 1.88 | 0.26 | 0.02 | -0.27 | 0.32 |
| BMD (Gruen zone 4) | total | - | - | - | - | 0.01 | -0.28 | 0.30 |
| BMD (Gruen zone 5) | 0 | 2.08 | 0.39 | 2.06 | 0.22 | 0.03 | -0.29 | 0.35 |
| BMD (Gruen zone 5) | 3 | 2.07 | 0.37 | 1.93 | 0.23 | 0.10 | -0.22 | 0.42 |
| BMD (Gruen zone 5) | 6 | 2.01 | 0.36 | 2.00 | 0.25 | 0.01 | -0.31 | 0.33 |
| BMD (Gruen zone 5) | 12 | 1.96 | 0.35 | 2.03 | 0.25 | -0.05 | -0.37 | 0.28 |
| BMD (Gruen zone 5) | 24 | 2.11 | 0.25 | 2.07 | 0.30 | 0.01 | -0.32 | 0.34 |
| BMD (Gruen zone 5) | total | - | - | - | - | 0.02 | -0.28 | 0.32 |
| BMD (Gruen zone 6) | 0 | 1.89 | 0.26 | 1.82 | 0.15 | 0.06 | -0.16 | 0.29 |
| BMD (Gruen zone 6) | 3 | 1.85 | 0.30 | 1.77 | 0.19 | 0.05 | -0.17 | 0.28 |
| BMD (Gruen zone 6) | 6 | 1.84 | 0.23 | 1.82 | 0.21 | 0.02 | -0.20 | 0.25 |
| BMD (Gruen zone 6) | 12 | 1.81 | 0.26 | 1.77 | 0.21 | 0.00 | -0.23 | 0.22 |
| BMD (Gruen zone 6) | 24 | 1.88 | 0.13 | 1.81 | 0.23 | 0.07 | -0.16 | 0.29 |
| BMD (Gruen zone 6) | total | - | - | - | - | 0.04 | -0.18 | 0.26 |
| BMD (Gruen zone 7) | 0 | 1.29 | 0.11 | 1.19 | 0.24 | 0.10 | -0.12 | 0.32 |
| BMD (Gruen zone 7) | 3 | 1.13 | 0.08 | 1.09 | 0.27 | 0.02 | -0.21 | 0.24 |
| BMD (Gruen zone 7) | 6 | 1.10 | 0.08 | 1.07 | 0.29 | 0.03 | -0.19 | 0.25 |
| BMD (Gruen zone 7) | 12 | 1.09 | 0.12 | 0.91 | 0.38 | 0.17 | -0.05 | 0.40 |
| BMD (Gruen zone 7) | 24 | 1.03 | 0.20 | 0.96 | 0.30 | 0.08 | -0.14 | 0.31 |
| BMD (Gruen zone 7) | total | - | - | - | - | 0.08 | -0.12 | 0.28 |
The widest range (not statistically significant) in BMD between and inside the groups was reported in the proximal Gruen zones 1 and 7 during the follow-up. SD for BMD in both groups in Gruen zones 1 and 7 was 7-19%, while in Gruen zones 2-6, it was about 2-7%. The greatest decrease in BMD values was noticed in Gruen zone 7. This decrease was up to 15% in the clodronate group, and up to 20% in the placebo group from baseline. In the other Gruen zones (2-6), the decrease in BMD was about 5% from baseline. Partial recovery was seen in both clodronate and placebo groups in Gruen zones 2-6, but only in the clodronate group in Gruen zone 7 and in neither group in Gruen zone 1 (Fig. 2). There were no statistically significant differences between the study groups in the intra-sample medial-lateral plateau BMDs (data not shown).
3.2. Effect of Clodronate Rinsing on the Stem Migration
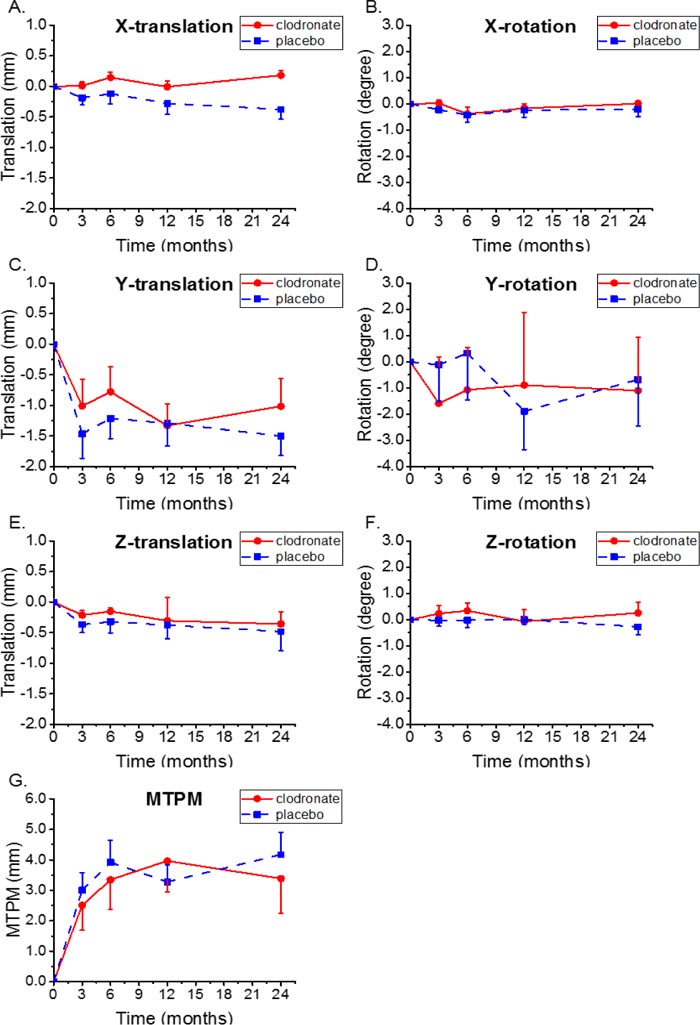
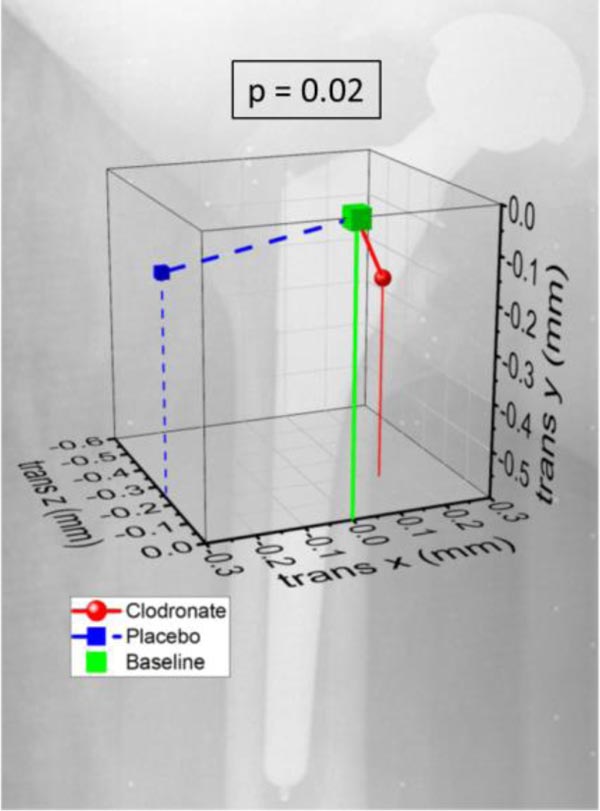
There were wide individual variations in RSA-measured micromovement in both study groups at all time-points. Micromovement was generally greatest during the first 3 months. There were no statistical differences in MTPM or x-, y- and z-translations of the femoral stem during the 2 year follow-up period between the study groups (Fig. (3). The migration values, estimated as differences between study groups, were lower in the clodronate group over the entire period of 0-24 months: MTPM -0.40 (CI -2.50-1.70), x-translation -0.16 mm (CI: -0.60-0.28), y-translation -0.33 mm (CI: -1.38-0.71), and z-translation -0.05 mm (CI: -0.60-0.50); values for rotations were not defined. The maximal translation in the time frame of 3 to 24 months was 0.14 mm (SD 0.27) to medial in the clodronate group and 0.29 mm (SD 0.43) in the lateral direction in the placebo group (p = 0.02) (Fig. 4).
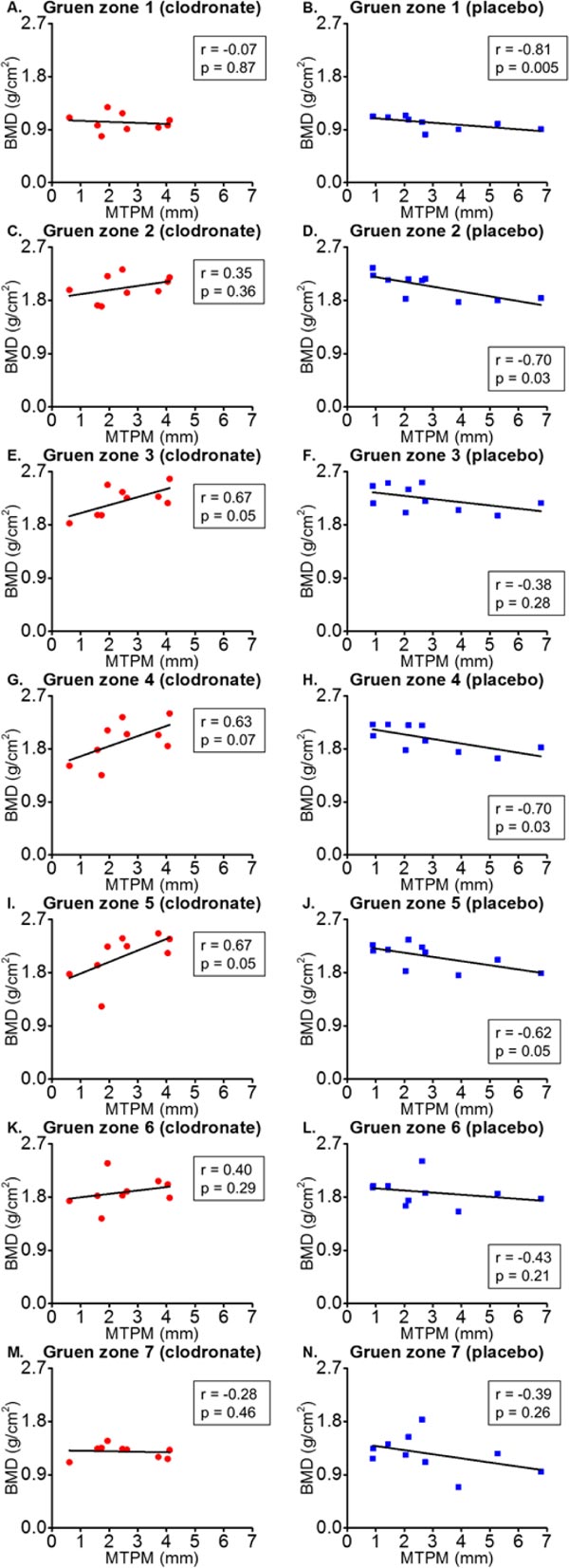
3.3. Correlation between BMD and the Stem Migration
There was a positive correlation between baseline BMD values and greatest MTPM values after 3 months in Gruen zones 3 (r=0.67, p=0.05), 4 (r=0.63, p=0.07) and 5 (r=0.67, p=0.05) in the clodronate group, a negative correlation in Gruen zones 1 (r=-0.81, p=0.005) 2 (r=-0.70, p=0.03), 4 (r=-0.70, p=0.03) and 5 (r=-0.62, p=0.05) in the placebo group. (Fig. 5).
3.4. The Effect of Clodronate on the Clinical Outcome
HHS increased up to the 12-month follow-up point in both the clodronate and the placebo groups, after which no significant improvement was noticed. There were no significant differences between the study groups in HHS during the 24-month follow-up period. (Table 1).
4. DISCUSSION
In this placebo-controlled, randomized study, we were able to show that the intraoperative local administration of clodronate may have some beneficial effects on the integration of the prosthesis. Due to the small study group sizes, when comparing the periprosthetic BMD, we were only able to achieve a level of significance in the Gruen zone 3 (time and group interaction). In that zone, BMD decreased less in the clodronate group than in the placebo group during the 2-year follow-up period. We also noted that both groups had greater periprosthetic bone loss in the proximal femur, areas of the lesser trochanter and trochanter major. There was also the greatest individual variation in these areas, which showed apparent differences between groups without statistical significance. Our results are parallel to those presented in previous studies in which bisphosphonate treatment has been shown to reduce periprosthetic bone loss. Generally, the greatest bone loss occurred in the proximal Gruen zones 1 and 7 [31, 32] and the least in Gruen zone 5. Also, the greatest benefits of bisphosphonates, including risedronate, etidronate and alendronate, were usually seen in the proximal femur [33-35]. Several studies have shown that the greatest periprosthetic bone loss occurs within the first three to six post-operative months and that the effects of bisphosphonates were mostly seen in the first six months to one year. After this period, the amount of bone resorption was minor [33-35]. In the main load-bearing areas, Gruen zones 6 and 7, the effects have been shown to last for as long as six years postoperatively [36].
Previously, Trevisan et al. found that weekly intramuscular clodronate treatment for 12 months after cementless THA reduced periprosthetic bone loss in Gruen zones 2 and 6 only at 12 months postoperatively [15]. Although clodronate has analgesic and anti-inflammatory responses compared to other bisphosphonates [13], its efficacy may still be minor compared to others. In our study, the absolute values for BMD were used in statistical analyses, and not the relative values. This may have an effect when analyzing changes from the baseline [37]. Vickers et al. suggested that the use of percentage change should be avoided, as it has less statistical efficiency [38].
We found there was a significant two-fold lateral translation in the placebo group compared to the medial translation of the clodronate group, with a maximal translation at 3-24 months. Although there was less migration in the clodronate group than in the placebo group, there was no statistical significance in the MTPM or translations of femoral stem over the 2 year follow-up period. However, we used this 3-24 month period to examine migration after the early stabilization phase. Migration behaved similarly in both groups during the entire follow-up period, and no continuous migration was observed. It has previously been noted that increased early x-translation as well as subsidence correlates with aseptic loosening and increased risk of prosthesis revision [2]. The absolute difference between our study groups remained quite small and we could only speculate its clinical significance.
The ability of the bisphosphonates to reduce x-translation of the femoral stem has not been demonstrated in previous studies. Only Friedl et al. have reported a trend of decreased subsidence of femoral stem with intravenous infused zoledronic acid [23]. However, encouraging evidence on the benefits of bisphosphonates has been reported earlier with acetabular components after THA [22, 23] and tibial components after TKA [39, 40].
According to these previous clinical studies, the local administration of bisphosphonates has been shown to be effective [22, 40] and systemic adverse effects were not seen [41, 42], which is in agreement with the findings of our study. After the positive results of the peroral bisphosphonate in the integration of the prosthesis, our expectations were high with this intra-operative local rinsing, as this method of administration enables a stronger concentration of the bisphosphonate access to the areas to which it is aimed, and thus longer-lasting benefits can be expected [43, 44]. Whether due to the small study group or to the first-generation bisphosphonate, clodronate was not potent enough to demonstrate a stronger correlation for the better integration of stem.
With regards to the biomechanics of the proximal femur, the physiological load can be roughly divided into two types of forces, compression and tension, acting in different directions, in a clockwise torque power manner around the femoral head. Compression forces are generated on the medial side of the femoral shaft, while tension on the lateral side. The stresses are predominantly compressing, when ligamentous and muscular forces are included in the normal situation [45, 46]. Bone is found to be stronger in compression than in tension; also the trabecular architecture in the proximal femur is organized to prevent these forces [47]. This balance may be changed after THA due to the non-anatomical repair of ligament structures, changes in hip off-set and changes in bone structure [48]. Because of the great periprosthetic bone loss in the proximal femur, the compression force could push the femoral stem in a lateral direction, as we observed in our placebo group. By preventing periprosthetic bone loss with bisphosphonate treatment, the bone maintains its strength to resist external forces and the femoral stem will probably remain fixed. Furthermore, theoretically better, but not significant, periprosthetic BMD maintenance in the placebo group than in the clodronate group in Gruen zone 1 may have been caused by a higher pressure in the lateral direction, while in Gruen zone 3 where the pressure was diminished due to the moment effect of the stem, it resulted in periprosthetic bone loss.
We found a negative correlation between initial postoperative BMD and the migration rate of the femoral stem in the placebo group. Similar results have been found in some clinical trials [49, 50]. Compared to the placebo group, the clodronate group showed an opposite effect in its relation between the initial postoperative BMD and migration of the femoral stem: low BMD values correlated with a low migration rate. We did not find any previous studies to support these findings. The negative correlation of BMD to MTPM in the clodronate group might be explained by the fact that, even if there is stem migration, it only activates bone resorption, but when there is clodronate attached to the bone minerals, activated osteoclasts will end up in apoptosis. The bone seems not to be resorbed but strengthened, as we have demonstrated here [8, 9]. The alternative explanation could be that clodronate may improve BMD in the proximal femur but either it does not increase the proximal femur BMD enough to have an effect on short-term subsidence or it does not affect the bone-stem interface enough.
The limitation of our study was the size of the study population; only 9 patients were included in the clodronate group and 10 in the placebo group. Thus, the statistical power remains low, although the accuracy of the RSA-method is very high [28, 51, 52]. This study has been planned as a pilot study to obtain preliminary results which will serve as a platform for further research with a larger sample size. Another limitation was the short follow-up period. Although there is evidence that early migration during the first 2 postoperative years predicts aseptic loosening of the prosthesis, it would be necessary to carry out randomized controlled trials with longer follow-up periods.
We did not find out any differences between study groups in the most clinically relevant RSA parameters: MTPM, subsidence or internal rotation of the femoral stem. However, in this small pilot study, we noticed some interesting findings with stem lateral migration and periprosthetic bone quality. The relation of these findings to aseptic loosening of the prosthesis is not entirely clear. They are still promising findings however, and worth carrying out a larger study intervention to establish their importance.
CONCLUSION
In conclusion, based on our study results, it is difficult to evaluate whether or not clodronate rinsing prevents periprosthetic bone loss and further stabilizes the stem. Larger studies with longer follow-up periods are needed.
AUTHORS’ CONTRIBUTIONS
Jukka Kiuttu – acquisition, analysis and interpretation of data, drafting the paper, Hannu-Ville Leskelä – acquisition of data and critical revision of the paper, Olli Yrjämä – acquisition of data and critical revision of the paper, Pasi Ohtonen – analysis of data and critical revision of the paper, Petri Lehenkari – research design and critical revision of the paper and Maarit Valkealahti – research design, interpretation of data and critical revision of the paper. All authors have read and approved the final submitted manuscript.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study protocol was approved by the Oulu University ethical committee 100/2000 and was in accordance with the legal requirements for medical research (488/1999).
HUMAN AND ANIMAL RIGHTS
No animals were used in this research. All human research procedures were followed in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2013.
CONSENT FOR PUBLICATION
All patients gave their written informed consent before becoming involved in the study.
STANDARDS OF REPORTING
CONSORT guidelines and methodology have been followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
ACKNOWLEDGEMENTS
For altruistic English proofreading, we thank Ph.D. MD. Michael Spalding.


